Archives of Clinical and Experimental Surgery
"Archives of Clinical and Experimental Surgery" is a peer-reviewed publication covering the entire field of surgical sciences. The Journal is published quarterly and is dedicated to virtually all fields of surgical sciences. Experimental and clinical studies, as well as scientific reviews are welcome for consideration in the Journal. Furthermore, the Journal publishes also, short or case reports and finally commentary letters.
Submit Manuscript
Fast Editorial Execution and Review Process (FEE-Review Process):
Archives of Clinical and Experimental Surgery is participating in the Fast Editorial Execution and Review Process (FEE-Review Process) with an additional prepayment of $99 apart from the regular article processing fee.�Fast Editorial Execution and Review Process is a special service for the article that enables it to get a faster response in the pre-review stage from the handling editor as well as a review from the reviewer. An author can get a faster response of pre-review maximum in 3 days since submission, and a review process by the reviewer maximum in 5 days, followed by revision/publication in 2 days. If the article gets notified for revision by the handling editor, then it will take another 5 days for external review by the previous reviewer or alternative reviewer.
Acceptance of manuscripts is driven entirely by handling editorial team considerations and independent peer-review, ensuring the highest standards are maintained no matter the route to regular peer-reviewed publication or a fast editorial review process. The handling editor and the article contributor are responsible for adhering to scientific standards. The article FEE-Review process of $99 will not be refunded even if the article is rejected or withdrawn for publication.
The corresponding author or institution/organization is responsible for making the manuscript FEE-Review Process payment. The additional FEE-Review Process payment covers the fast review processing and quick editorial decisions, and regular article publication covers the preparation in various formats for online publication, securing full-text inclusion in a number of permanent archives like HTML, XML, and PDF, and feeding to different indexing agencies.
2022, Volume 11, Issue 8
Case Report
-
Comprehensive Treatment of a Rare Primary Malignant Pericardial Mesothelioma: A Case Report and Literature Review
Chunyu Liu, Bin Zhao, Weiwei Cheng, Dongliang Ma, Xing Wei and Shunye Zhang*
Arch Clin Exp Surg. 2022; 11(8): 1 - 8
Perspective
-
Oncological Outcomes of Patients Undergoing Cancer Surgery
Arch Clin Exp Surg. 2022; 11(8): 1 - 1
Perspective
-
Outcomes and Resuscitation of a Patient in Damage Control Surgery
Arch Clin Exp Surg. 2022; 11(8): 1 - 1
Opinion Article
-
Use of Electrosurgery during Outpatient Treatments
Arch Clin Exp Surg. 2022; 11(8): 1 - 1
Commentary
-
Treatment Involved in Outpatient Surgery and its Procedures
Arch Clin Exp Surg. 2022; 11(8): 1 - 1
2022, Volume 11, Issue 6
Commentary
-
Techniques and Procedures Involved in Plastic Surgery
Arch Clin Exp Surg. 2022; 11(6): 1 - 1
Perspective
-
Surgical Treatment and Diagnosis of Colorectal Surgery
Arch Clin Exp Surg. 2022; 11(6): 1 - 1
Commentary
-
Impact of Surgical Trauma on Patients
Arch Clin Exp Surg. 2022; 11(6): 1 - 1
Opinion Article
-
Role of Postoperative Care in Surgical Patients
Arch Clin Exp Surg. 2022; 11(6): 1 - 2
Perspective
-
Types and Criteria of Intestinal Bypass Surgery
Arch Clin Exp Surg. 2022; 11(6): 1 - 1
2022, Volume 11, Issue 4
Perspective
-
Principles and Benefits of Bloodless Surgery
Arch Clin Exp Surg. 2022; 11(4): 1 - 2
Commentary
-
Note on Immunosurgery in Surgical Sciences
Arch Clin Exp Surg. 2022; 11(4): 1 - 1
Perspective
-
Brief Note on Neurosurgery and its Methods
Arch Clin Exp Surg. 2022; 11(4): 1 - 2
Opinion Article
-
Note on Surgical instruments and its Classification
Arch Clin Exp Surg. 2022; 11(4): 1 - 2
Case Report
-
Laparoscopic Cholecystectomy in a Patient with Lumbar Peritoneal Shunt (LP Shunt): A Case Report
Musharraf Husain, Tajamul Rashid*, Sanjana Agarwal, Sonali Ohri and Nikhat Sartaj
Arch Clin Exp Surg. 2022; 11(4): 1 - 3
2022, Volume 11, Issue 5
Perspective
-
Note on Perceptions Regarding Endarterectomy
Arch Clin Exp Surg. 2022; 11(5): 1 - 1
Opinion Article
-
Coronary Artery Bypass Surgery and its Efficacy in Surgical Sciences
Arch Clin Exp Surg. 2022; 11(5): 1 - 1
Research Article
-
A Comparative Study of One-stage, Two-stage and Three-stage Posterior Sagittal Anorectoplasty (PSARP) in Female Anorectal Malformations (ARM)
Ashok Laddha, Brijesh Lahoti, Maneesh Jholeya, Aditi Deewan*, Shashi Shankar Sharma, Ram Mohan Shukla, Pooja Tiwari and Yashaswi Choudhury
Arch Clin Exp Surg. 2022; 11(5): 1 - 5
Commentary
-
A Brief Note on Debridement and its Classification
Arch Clin Exp Surg. 2022; 11(5): 1 - 2
Research Article
-
Evaluation of the Association between Breast Imaging Reporting and Data System for Ultrasonography (BIRADS) and Histopathology in Patients of Lump nn Breast: An Observational Study
Samprati Dariya*, Sonia Moses, Sachin Verma, Prakhar Chaudhary and Sarvagya Jain
Arch Clin Exp Surg. 2022; 11(5): 1 - 7
2022, Volume 11, Issue 3
Perspective
-
A Detailed Note on Hand Surgery and its Types
Arch Clin Exp Surg. 2022; 11(3): 1 - 2
Commentary
-
Effects and Applications of Laser Surgery
Arch Clin Exp Surg. 2022; 11(3): 1 - 2
Perspective
-
Surgical Incision and its Classification in Surgery
Arch Clin Exp Surg. 2022; 11(3): 1 - 1
Case Report
-
Prepyloric Perforation Following Blunt Trauma Abdomen
Sarvagya Jain*, Prakhar Chaudhary, Lavanya Kelam, Arvind Ghanghoria and Hemant Thanna
Arch Clin Exp Surg. 2022; 11(3): 1 - 4
Short Communication
-
Validation of P-POSSUM Score as Mortality Predictor in COVID-19 Positive Patients Submitted to Emergency Digestive Surgery
Zoilo Madrazo*, Javier Osorio, Sebastian Videla and Sebastiano Biondo
Arch Clin Exp Surg. 2022; 11(3): 1 - 2
2022, Volume 11, Issue 2
Case Series
-
Abdominal Complications of VP Shunt in Pediatric Patients
Sarvagya Jain*, Shashi Shankar Sharma, Ram Mohan Shukla, K Lavanya, Hemant Thanna and Saksham Kumar
Arch Clin Exp Surg. 2022; 11(2): 1 - 5
Research Article
Perspective
-
Classification and Types of Grafting in Surgery
Arch Clin Exp Surg. 2022; 11(2): 1 - 1
Perspective
-
Note on Organ Transplantation and Types of Donor
Arch Clin Exp Surg. 2022; 11(2): 1 - 1
Commentary
-
Types and Risks Associated with Cardiac Surgery
Arch Clin Exp Surg. 2022; 11(2): 1 - 1
2021, Volume 10, Issue 8
Brief Commentary
-
Open Appendectomy Surgery General Procedure
Nakajima Maeno
Arch Clin Exp Surg. 2021; 10(8): 0 - 1
Commentary
-
Complications and Risks Involved in Arthroscopy
Filippo Columbano
Arch Clin Exp Surg. 2021; 10(8): 1 - 2
Editorial
-
A Note on Intra-Operative Phase in Surgery
Simon Samuel
Arch Clin Exp Surg. 2021; 10(8): 1 - 2
Editor Note
-
Anti-Cancer Compounds with Clinical Efficacy
Benning Houston
Arch Clin Exp Surg. 2021; 10(8): 1 - 2
Research
-
Infantile Hypertrophic Pyloric Stenosis (IHPS): Demographic, Clinical and Biochemical
Profile and Outcome at a Tertiary Care Hospital
Parveen Kumar
Arch Clin Exp Surg. 2021; 10(8): 1 - 4
2021, Volume 10, Issue 9
Commentary
-
Deep Brain Stimulation
Arch Clin Exp Surg. 2021; 10(9): 1 - 2
Editorial
-
A Note on Anterior Cruciate Ligament (ACL) Surgery
Arch Clin Exp Surg. 2021; 10(9): 0 - 1
Editorial
-
Turbinectomy To Reduce the Size of the Inferior Turbinates
Arch Clin Exp Surg. 2021; 10(9): 1 - 2
Commentry
-
Complications in Anal Fistula
Arch Clin Exp Surg. 2021; 10(9): 0 - 1
Research
-
Post-Operative Wound Complications Following Abdominal Surgery in Children: A Single Centre Experience
Arch Clin Exp Surg. 2021; 10(9): 1 - 5
2021, Volume 10, Issue 6
Short Communication
-
The Beauty of Plastic Surgery
Arch Clin Exp Surg. 2021; 10(6): 1 - 2
Short Communication
-
Endocrine system and its functions
Arch Clin Exp Surg. 2021; 10(6): 1 - 2
Editorial
-
Thoracic surgeries
Arch Clin Exp Surg. 2021; 10(6): 1 - 1
Editorial
-
Orthopedic surgery
Arch Clin Exp Surg. 2021; 10(6): 1 - 1
Short Communication
-
Troubleshooting Surgical Staple Failure
Howard Blum
Arch Clin Exp Surg. 2021; 10(6): 1 - 2
2021, Volume 10, Issue 7
Short Communication
-
Methods employed in Neurosurgery
Arch Clin Exp Surg. 2021; 10(7): 1 - 2
Short Communication
-
Methods in Neurosurgery
Arch Clin Exp Surg. 2021; 10(7): 1 - 2
Editorial
-
A Randomized Controlled Study of Utilizing Far Bloc to Decrease Hot Flashes in
Postmenopausal Women
Arch Clin Exp Surg. 2021; 10(7): 1 - 2
Editorial
-
Surgical Strengths: Results of a Review Cross Country Investigation
Arch Clin Exp Surg. 2021; 10(7): 1 - 2
Commentary
-
Advance Methods and Techniques for Surgeries
Arch Clin Exp Surg. 2021; 10(7): 1 - 2
2021, Volume 10, Issue 5
Editorial
-
An Editorial on Colon and Rectal Surgery
Arch Clin Exp Surg. 2021; 10(5): 1 - 1
Editorial
-
Hernia Repair Surgery
Arch Clin Exp Surg. 2021; 10(5): 1 - 1
2021 Conference Announcement
-
2nd International Conference on Aesthetic Medicine
Arch Clin Exp Surg. 2021; 10(5): 1 - 1
Young Research Forum
-
13th International Conference on Transplantation and Medical Surgery
Arch Clin Exp Surg. 2021; 10(5): 1 - 1
Young Research Forum
-
26th International Conference on Cardiovascular and thoracic Surgery
Arch Clin Exp Surg. 2021; 10(5): 1 - 1
2021, Volume 10, Issue 11
Commentary
-
A Short Note on Ileal Pouch: Anal Anastomosis
Arch Clin Exp Surg. 2021; 10(11): 0 - 1
Editorial Note
-
A Note on Cochlear Implant
Arch Clin Exp Surg. 2021; 10(11): 0 - 1
Commentary
-
A Short Note on Arthrodesis
Arch Clin Exp Surg. 2021; 10(11): 0 - 1
Research
-
Assessment of Decompressive Surgeries as a Pain Relief in Case of Chronic Pancreatitis
Saikalyan Guptha A, Ravi Kumar H*, Sidduraj Sajjan, Siva Shankar, Sowmya C U and Yeshwanth S
Arch Clin Exp Surg. 2021; 10(11): 1 - 6
Research
-
Core Bone Diameter in an Organic Implant-Less Technique Affecting the Biomechanical Properties of the Anterior Cruciate Ligament Fixation; an In-Vitro Study
Mahdi Mohseni, Amir Nourani*, Hossein Korani, Hadi Moeinnia, Amirhossein Borjali, Narges Ghias and Mahmoud Chizari
Arch Clin Exp Surg. 2021; 10(11): 1 - 7
2021, Volume 10, Issue 10
Case Report
-
A Case Study of the Application of WEKA Software to Solve the Problem of Liver Inflammation
Arch Clin Exp Surg. 2021; 10(10): 1 - 13
Editorial
-
A Note on Cataract Surgery
Arch Clin Exp Surg. 2021; 10(10): 0 - 1
Commentary
-
A Short Note on Coronary Angioplasty
Arch Clin Exp Surg. 2021; 10(10): 1 - 2
Commentry
-
A Note on Eye Muscle Surgery
Arch Clin Exp Surg. 2021; 10(10): 0 - 1
Editorial Note
-
A Short Note on Debridement
Arch Clin Exp Surg. 2021; 10(10): 0 - 1
2022, Volume 11, Issue 7
Commentary
-
Predictive Methods for Surgical Time during Surgery
Arch Clin Exp Surg. 2022; 11(7): 1 - 1
Opinion Article
-
Clinical Practice in Plastic Surgery and its Sub Disciplines
Arch Clin Exp Surg. 2022; 11(7): 1 - 1
Opinion Article
-
Surgical Techniques and Complications Associated with Aortic Surgery
Arch Clin Exp Surg. 2022; 11(7): 1 - 2
Perspective
-
Surgical Procedures Involved in Orthopedic Surgery
Arch Clin Exp Surg. 2022; 11(7): 1 - 2
Commentary
-
Current Trends in Vascular Procedures and Treatments of Vascular Surgery
Arch Clin Exp Surg. 2022; 11(7): 1 - 2
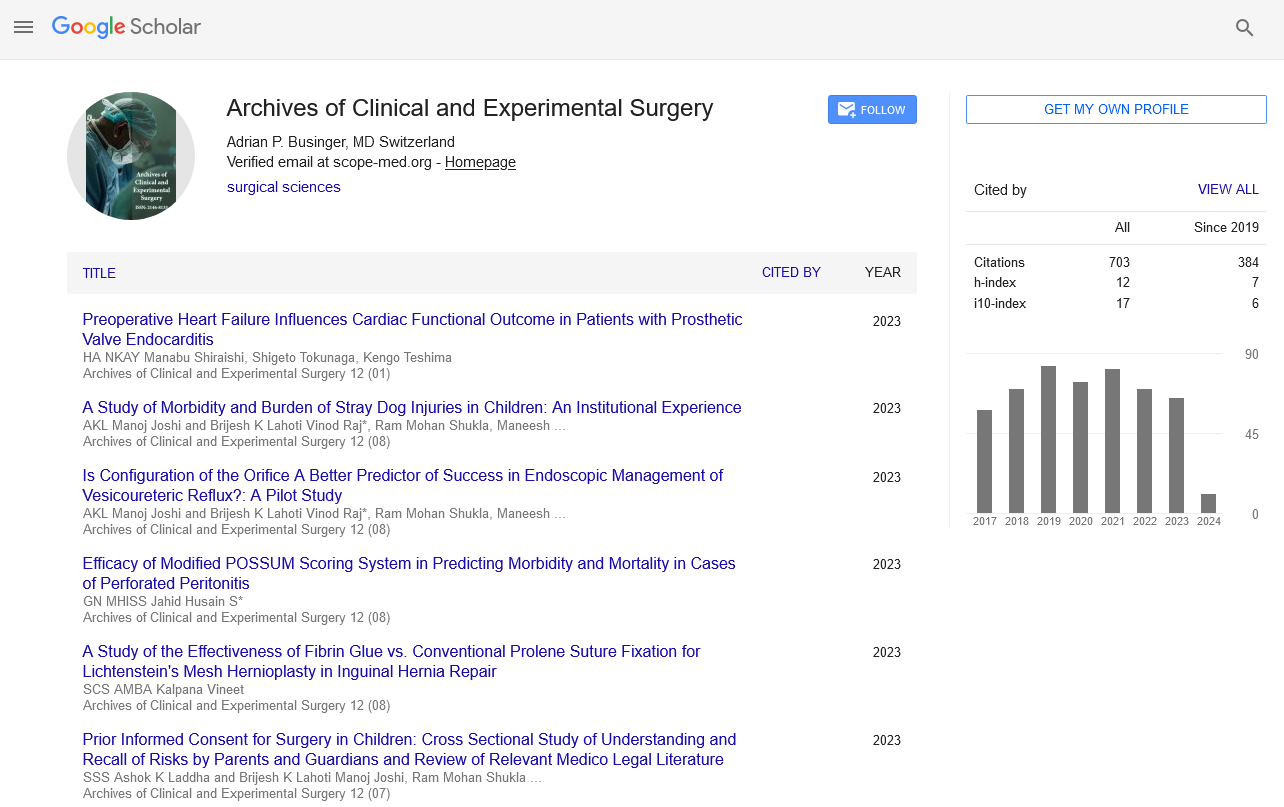
h-index
Articles published in Archives of Clinical and Experimental Surgery have been cited by esteemed scholars and scientists all around the world. Archives of Clinical and Experimental Surgery has got h-index 11 , which means every article in Archives of Clinical and Experimental Surgery has got 11 average citations.