Research Article - Archives of Clinical and Experimental Surgery (2024)
A Comparative Study on the Clinical Efficacy of Inflatable Mediastinoscopy Syncrhonized Laparoscopic Esophagectomy with Mckeown's Technique
Hai-Tao Wei1, Yang-Yang Liu2, Meng-Yao Wang2, Hai-Feng Zhang2, Bao-Li Hu2, Dong-Hong Zhang2, Xiao-Long Wang2, Lin-Lin Fan2, Xia Kang2 and Li Li1,2*2Department of Nursing and Health, Henan University, Henan, China
Li Li, Department of Thoracic Surgery, Huai He Hospital, Henan University, Henan, China, Tel: +86-13703786236, Email: 10210051@vip.henu.edu.cn
Received: 15-Mar-2024, Manuscript No. EJMACES-24-129765; Editor assigned: 18-Mar-2024, Pre QC No. EJMACES-24-129765 (PQ); Reviewed: 01-Apr-2024, QC No. EJMACES-24-129765; Revised: 08-Apr-2024, Manuscript No. EJMACES-24-129765 (R); Published: 15-Apr-2024
Abstract
Background: Inflatable mediastinoscopy synchronous laparoscopic radical esophagectomy is not widely used domestically and internationally. In order to explore the technological innovation and application effects of inflatable mediastinoscopy synchronous laparoscopic radical esophagectomy, our team summarizes our surgical experience and hopes to further promote its application in clinical practice.
Objective: To explore the technical innovation and application effect of inflatable mediastinoscope synchronous laparoscopic radical resection of esophageal cancer.
Methods: From January 2017 to December 2018, 120 patients with esophageal cancer admitted by the same surgical team were retrospectively analyzed. A total of 64 patients were randomly divided into two groups: The experimental group with inflatable mediastinoscopy and the conventional group with McKeown (control group). Preoperative baseline data, perioperative index, postoperative index, near and long term survival rate and other indexes of patients in 2 groups were recorded, and statistical analysis was performed.
Results: The operation was completed successfully in both groups. In the experimental group, the operation time was (116.26 ± 43.34) min, the intraoperative blood loss was (33.28 ± 19.78) ml, the lymph nodes were removed (32.77 ± 2.23), and the hospital stay was (9.62 ± 3.33) d. Esophagogastric anastomotic leakage occurred in 2 cases, recurrent laryngeal nerve injury in 1 case and chylous leakage in 1 case. All 120 patients were followed up until December 2023. None of the 120 cases had tumor recurrence and metastasis, and no death.
Conclusion: Synchronous laparoscopic radical resection of esophageal cancer with inflatable mediastinoscope has no postoperative chest complications, shorter operation time, less pain and faster recovery. It can be used as a new supplement to the mainstream McKeown surgery for radical resection of esophageal cancer, and has a good development prospect. It is recommended to actively promote its application in clinical practice.
Keywords
Inflatable mediastinoscope synchronous laparoscopy; McKeown operation; Radical resection of esophageal carcinoma; Comparative study
Introduction
Esophageal cancer is a prevalent malignancy that is observed globally, and within China, esophageal squamous cell carcinoma is particularly common, mostly associated with an unfavorable prognosis [1]. At present, surgery remains the primary therapeutic approach, particularly for patients in the initial and intermediate phases, which can achieve the purpose of radical treatment [2]. At present, surgery remains the primary therapeutic approach, particularly for patients in the initial and intermediate phases, which can achieve the purpose of radical treatment [3]. The evolution of surgical techniques for esophageal cancer resection has progressed from the initial left thoracotomy with a large incision using the sweet technique, to the adoption of the Ivor-Lewis technique involving a right thoracotomy and abdominal double incision. Subsequently, the McKeown technique emerged, which entails a triple incision in the neck, right thorax, and abdomen for esophageal cancer resection. Presently, the thoracoabdominal laparoscopic combined esophagectomy has become the prevailing technique for esophageal cancer resection. Transthoracic esophagectomy is widely utilized as the primary clinical approach for treatment. However, this procedure is associated with large surgical trauma, significant postoperative pain, high incidence of postoperative respiratory complications, and elevated perioperative mortality rate [4]. Moreover, the performance of transthoracic esophagectomy is associated with a lengthened duration of postoperative hospitalization, heightened hospital mortality rates, impeded post-surgical recovery, and potentially affected long-term patient survival rates.
At present, only a few hospitals in China carry out inflatable mediastinoscopy synchronized laparoscopic esophagectomy. Inflatable mediastinoscopy synchronized laparoscopic esophagectomy is a surgical technique employed for the purpose of dissecting, dissociating and excising the esophagus located within the mediastinum, and of excising entire mesoesophageal as well as lymph node. It has the potential to mitigate pleural cavity damage, alleviate postoperative pain, and facilitate expedited recovery following surgery. In addition, transmediastinal esophagectomy emerges as an optimal option for a subset of older individuals or those with compromised cardiopulmonary function due to its notable advantages, including a reduced duration of surgery, absence of chest trauma- induced pain, fewer postoperative complications and rapid recovery. In October 2018, a single-center study was original proposed by the surgical team from the Department of Thoracic Surgery at Huaihe Hospital of Henan University. The study aimed to investigate the efficacy of inflatable mediastinoscopy synchronized laparoscopic esophagectomy as a treatment for esophageal cancer. This proposal was based on the team’s extensive experience in performing inflatable mediastinoscopy synchronized laparoscopic esophagectomy for the treatment of esophageal cancer. To date, a total of 120 patients have been recruited for the study. Now, we summarize our surgical experience, hope to further promote the application in clinical, so that more patients with esophageal cancer can really benefit from it.
Materials and Methods
General information
From January 2017 to January 2018, 20 patients diagnosed with esophageal cancer and treated with minimally invasive laparoscopic esophagectomy by a consistent surgical team were included in the study. The study according to different surgical methods classified into two groups: The McKeown group consisting of 56 patients, and the inflatable mediastinoscopy synchronized laparoscopic esophagectomy group consisting of 64 patients. The criteria for inclusion in this study were as follows.
1) All enrolled patients were diagnosed as esophageal squamous cell carcinoma by preoperative gastroscopy and pathology.
2) All patients underwent either minimally invasive McKeown esophagectomy or inflatable mediastinoscopy synchronized laparoscopic esophagectomy procedures, which were successfully completed.
3) Patients were in tumor stage T1-2N0-1M0 or T3N1M0 (IIIa) without cervical lymph node enlargement and distant metastasis, or for surgically resectable T1-2 esophageal cancer. The presence of bulky type tumors in T3 was also excluded.
4) Patients had not undergone previous gastrointestinal tumor surgery or thoracic surgery, or patients with a history of thoracic surgery didn’t have dense adhesion of the pleural cavity, marginal lung function, and thoracic deformity. However, patients with a history of induction therapy were not contraindicated for surgery, and the evaluation of the tumor boundary is necessary.
5) The clinical data of the patients was complete.
The exclusion criteria for the patients in this study are as following.
1) Patients had tumor adjacent to the lower part of cardia and esophagus or gastroesophageal junction, which could be operated through single left thoracic approach.
2) Patients were converted to conduction minimally invasive esophagectomy or conventional transthoracic esophagectomy.
3) Patients were re-operation during the patient’s hospital stay.
4) Patients were absence of suitable gastrointestinal replacement organs.
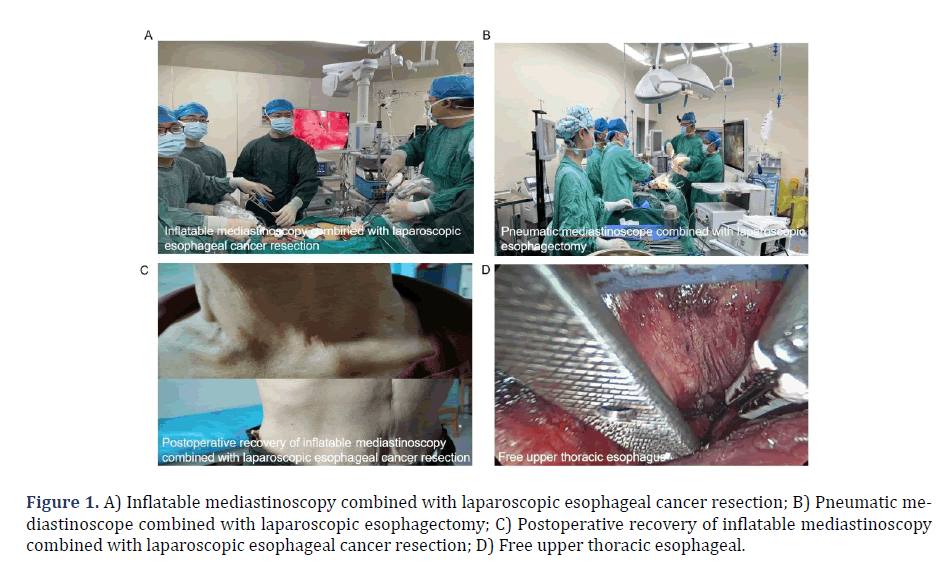
5) There were not sufficient clinical data for the patient. This study was approved by the Ethics Committee of Henan University,under the permission number HUSOM2023-348. All patients provided their informed consent by signing the necessary documentation. According to the above criteria, a total of 120 eligible esophageal cancer patients were enrolled in this study (Figures 1A,1B and 1C). All the enrolled patients successfully completed the surgery and no deaths occurred during the perioperative period.
Surgical method
Preoperative preparation: 100 ml of olive oil or 1000 ml of pure milk was ingested at 23:00 on the evening prior to the surgical procedure. In order to empty stomach contents as much as possible, a gastric tube was introduced during the early morning on the day of the surgical procedure. 600 ml of plasma was prepared and preoperative cleansing enema was performed. A single stomach tube, two duodenal nutritional tubes, and a circular stapler with size of 33 and 26 were prepared. 6-8 sets of linear cutter staplers with a length of either 75 mm or 100 mm, 12-15 purple Home-lock plastic clips, and an operating table with detachable legs were prepared.
The operation position: Put “person” figure open leg supine position, and placed in the dorsal elevated position. The following equipment were also prepared. 1) disposable puncture device: Two 5-mm and three 10-mm; 2) 4-0 and 3-0 absorbable sutures; 3) mediastinoscopy and laparoscopy, preferably of ultra-high definition, and two pneumoperitoneums, preferably Kangmei high-end machines; 4) one multi-channel protective sleeve for neck; 5) prepare an ultrasonic scalpel, an Medtronic energy platform mainframe, and a matching vertical card cutting head; 6) the mediastinal instruments used during surgery may need to be rapidly disinfected.
Surgical procedure: Under general anesthesia, single- lumen intubation was performed in the supine position with leg apart. Positive pressure ventilation was given to both lungs. During the surgical procedure, the vital signs of pulse, blood pressure, and oxygen saturation were continuously monitored. The shoulder of the patient was appropriately cushioned, and the positioning of the head to the right in order to adequately expose the left side of the neck. The patient’s head raised to 30° and maintained in the dorsal elevated position. The arms were positioned alongside the upper body, the extension tube for the deep vein puncture of the neck was located at the feet and the legs were split at 45°. Iodophor was used to sterilize the skin at the neck and abdominal surgical sites, the area from the upper to lower lip, along both sides of the trapezius muscle extending to approximately 1/3 of the left upper arm, along the midaxillary line on both sides extending to approximately 1/3 of the upper leg, and the surgical spreading sheets and drapes. Two sets of high-definition machines were placed on the right side of the bed and the distal end of the bed. The instrument nurses were also divided into two groups, specifically positioned at the cephalosome and abdominal portions. The preparation of surgical equipment, including two 5 mm lenses and mediastinal retractors, was required for the cephalosome part. The abdominal group required a set of three 10 mm lenses. The surgeons were divided into two distinct groups, one group performed neck mediastinoscopy procedures, while the other group performed abdominal laparoscopic surgeries concurrently, in the cervical mediastinoscopy group, there were two doctors; one surgeon, one assistant, and one laparoscope hand; The abdominal laparoscopy group consisted of two doctors, one surgeon and one laparoscope.
Inflatable mediastinoscopy synchronized laparoscopic esophagectomy
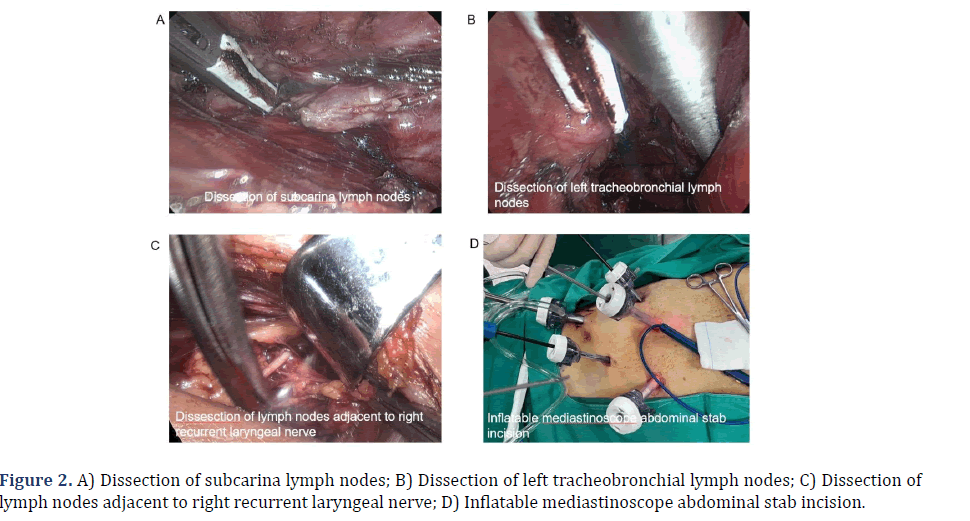
Mediastinal operation group: A 4 cm incision was performed with a curvature aligned parallel to the clavicle, situated 2 cm above the left clavicle in the neck. The anterior edge of the sternocleidomastoid muscle was dissected layer by layer to expose the left recurrent laryngeal nerve, clear the perineural lymph nodes, and to achieve complete mobilization of the neck esophagus, which was then appropriately labeled (Figure 1D). A neck incision protector was placed, followed by the closure of the lid of the designated protector. Subsequently, two specialized trocars with a diameter of 5 mm were inserted and connected to a CO2 gas. The mediastinum was then insufflated with CO2 gas at a pressure of 10-12 mmHg, resulting in the induction of an artificial mediastinal emphysema. A 30° scope with 5 mm in diameter, Liga Sure (model: LF1537), and a specially made superior mediastinal retractor were inserted through 2 trocars, respectively. Firstly, along the left side of the esophagus, the mediastinal retractor was assisted to closely dissect the esophagus under the carina or even in front of the pericardium. During the operation, the surgeon should try to dissect the lower pulmonary vein as close as possible to the level of the lower pulmonary vein, so as to minimize the challenges associated with mobilizing the esophagus from the abdominal cavity and facilitate the thorough cleaning of the subcarinal lymph nodes (Figure 2A). Meanwhile, it was crucial to exercise caution in order to safeguard the thoracic duct in the deeper anatomical structures. Then, the tracheoesophageal groove on the right side of the esophagus was continued in a downward direction, and the periesophageal and tracheal tissues and lymph nodes were bluntly dissociate and excised. The lymph nodes and adjacent tissues were repositioned laterally to the esophagus, extending as far as feasible, and afterwards dissected downwards until reaching the inferior border of the left major bronchus. The surgeon was advised to exercise caution while performing dissection of the lymph nodes located posterior to the primary trachea, as well as the tissues situated on the left side of the primary trachea (Figure 2B). Additionally, it was imperative to prioritize the preservation of the azygos vein and the tracheal membrane during the procedure. Additionally, the left recurrent laryngeal nerve was observed and the direction of the nerve was determined. The nerve was bluntly dissected anteriorly to the esophagus, and the surrounding tissue was excised. The nerve was anatomically juxtaposed with the left recurrent laryngeal nerve in close proximity to the esophagus, and was then dissected till reaching the beginning of the left recurrent laryngeal nerve at the inferior border of the aortic arch. Finally, the left recurrent laryngeal nerve was dissected out with sharp endoscopic scissors, and the surrounding tissues and lymph nodes were left on the esophagus. The surgeon completely separated the nerve from the esophagus at the origin of the left recurrent laryngeal nerve, and proceeded to dissociate the esophagus in a downhill direction until further dissociation was not possible. At the same time, the presence of the right recurrent laryngeal nerve was detected, and only the lymph nodes next to the right recurrent laryngeal nerve and the surrounding soft tissue were excised (Figure 2C). Esophageal mobilization was not performed because the adequate mobilization of the esophagus achieved through the left neck incision. The procedure of recurrent laryngeal nerve lymph node dissection had been successfully performed.
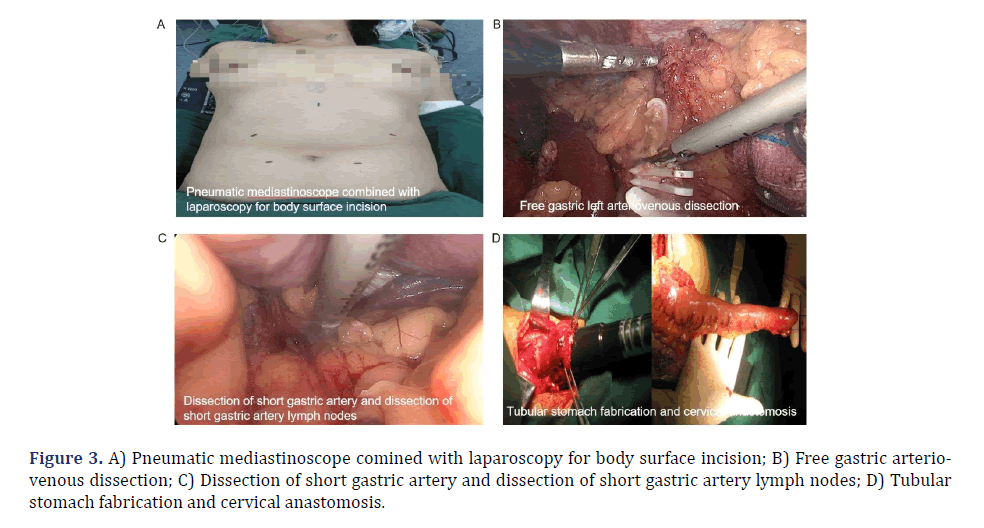
Figure 1: A) Inflatable mediastinoscopy combined with laparoscopic esophageal cancer resection; B) Pneumatic mediastinoscope combined with laparoscopic esophagectomy; C) Postoperative recovery of inflatable mediastinoscopy combined with laparoscopic esophageal cancer resection; D) Free upper thoracic esophageal.
Laparoscopy operation group (synchronized mediastinoscopy surgery): The site of puncture was located 1 cm superior to the umbilicus, and CO2 gas was introduced into the abdominal cavity at a pressure range of 10 to 14 mm Hg in order to create an artificial pneumoperitoneum. There were five laparoscopic surgical incisions (Figures 2D and 3A): 1) A laparoscopic observation port was created by making a 2 cm incision located 1 cm superior to the umbilicus. 2) Two incisions, one 1cm and the other 5 mm, were created above the previously formed apertures described in step 1. 3) The primary surgical incision was made at a distance of 3 cm from the paraumbilical area. 4) A 1 cm incision was made just below the right costal margin. 5) A 5 mm incision was performed below the xiphoid process, serving as an additional surgical incision. Gastric and abdominal lymph node dissection was performed under conventional laparoscopy. The gastrocolic ligament, spleen-stomach ligament, hepatogastric ligament, phrenogastric ligament and left gastric artery were dissected and dissociated under laparoscopy (Figure 3B). The left gastric vessel was ligated with a Home-lock clip and the associated lymph nodes were dissected (Figure 3C). The mediastinal retractor was employed to facilitate the visualization and mobilization of the lower esophagus up to the carina. A vertical incision measuring 5 cm was performed below the xiphoid process. This incision allowed for the extraction of drainage tubes from the esophagus, cardia, stomach, and mediastinum out of the abdomen, and then a 3-4 cm wide tubular stomach was made parallel to the greater curvature of the stomach with a cutting stapler. A gastric tube and nutrition tube were inserted, and the abdominal incision was closed using sutures.
Cervical anastomosis: A purse-string suture was em- ployed to secure the proximal neck of the esophagus, followed by the insertion of a stapler head pad and cut of the esophagus. A surgical procedure involved creating a 5 cm incision beneath the middle xiphoid process in the upper abdominal region, followed by the extraction of the esophagus and stomach from the abdominal cavity. The blood supply of the stomach wall was satisfactory. The stapler was excised at the cardia, and a tubular stomach was conducted 5 cm from the greater curvature. The right gastroepiploic artery was retained, the seromuscular layer was strengthened locally, and the stomach was sutured within the abdominal cavity. The tubular stomach was sent to the neck via the esophageal bed, and a distinct incision was performed at the lower portion of the stomach. A stapler was utilized to perform an anastomosis between the fundus of the stomach and the cervical esophagus, and additional sutures were employed to strengthen the incision made in the neck-stomach region (Figure 3D). The tubular stomach was suspended and fixed with several stitches. A nasogastric tube and jejunostomy tube were inserted via the nasal route. After inspection, it was observed that there was no evidence of ongoing hemorrhaging within the abdominal cavity and neck. Once the equipment and gauze were thoroughly inspected for accuracy, the abdominal drainage tube was carefully inserted, followed by suturing abdomen layer by layer. A drainage tube was inserted subcutaneously beneath the skin of the neck, and the incision on the neck was closed using sutures.
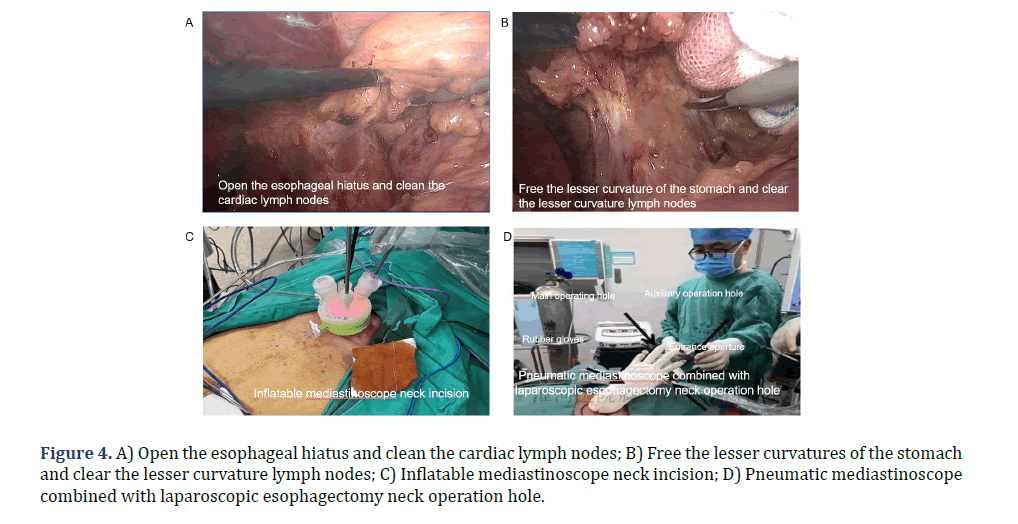
Control McKeown group: The patient was placed in the left lateral position at a 30° anterior tilt. Standard disinfection procedures were followed, and the patient was covered with sterile surgical drapes and towels. A 1.5 cm incision was made at the 7th intercostal space along the right mid-axillary line, followed by the insertion of a thoracoscope to visualize the thoracic cavity. In order to insert the operating instruments, three incisions were made. The first incision, measuring 0.5 cm, was performed in the 5th intercostal space beneath the right subscapular corner. The second incision, measuring 1.5 cm, was made in the 9th intercostal space along the posterior axillary line. Lastly, a 0.5 cm incision was created in the 4th intercostal space along the anterior axillary line insertion device. The posterior mediastinal pleura was incised with an electric hook, allowing for exploration of the tumor. The posterior superior mediastinal pleura was incised using an electric hook and an ultrasonic scalpel. Dissecting and isolating the right recurrent laryngeal nerve by hollowing out method, along with the removal of the lymph nodes in close proximity to the right recurrent laryngeal nerve. The distal and proximal terminations of the Azygos vein arch were mobilized and secured with double Home-lock clips and were severed. The dissection of the esophagus and tumor was performed, followed by the mobilization of the esophagus towards the superior thoracic aperture and its placement into an esophageal sleeve. Next, the dissection of the esophagus continued in a downward direction until reaching the esophageal diaphragmatic hiatus. The esophagus was anteriorly displaced, followed by the dissection and mobilization of the left recurrent laryngeal nerve, along with the subsequent removal of the left recurrent laryngeal nerve para-lymph nodes. The subcarinal lymph nodes were examined, and in the event of enlargement, they were excised with careful consideration given to preserving the integrity of the tracheobronchial membrane. A thoracic drainage tube was inserted adjacent to the esophageal bed, and subsequent closage of the chest incision was performed. The patient rolled over to supine position with a 30° elevation of the chest. The left neck and upper middle abdomen were re-disinfected, followed by the placement of a sterile drape. A 1.5 cm transverse incision was made above the umbilicus. After pneumoperitoneum was established, a laparoscope was introduced into the abdominal cavity to conduct an exploration. A pair of incisions were performed along the midline and on either side of the upper abdomen to facilitate the insertion of gripping forceps, an electric hook, and an ultrasonic scalpel. The ultrasonic scalpel was employed for the purpose of dissecting the greater curvature of the stomach and the short gastric arteries (Figure 4A). The gastrosplenic ligament was dissected to expose the cardia. It was important to prioritize the preservation of the right stomach vascular arch during the surgery. After the lesser omentum was incised, the left stomach artery was dissected and mobilized, and its base was occluded with a double Home-lock clip. Subsequently, the artery was transected using an ultrasonic scalpel. The dissociation of the minor curvature of the stomach was performed (Figure 4B), followed by dissection of the lesser omentum in the direction towards the pylorus and extending up to the esophageal hiatus. An oblique incision was made at the medial border of the left sternocleidomastoid, and all layers of neck muscles were incised. The esophagus was dissected along the medial aspect of the vascular sheath in the left neck, allowing for mobilization of the esophagus via the esophageal bed and subsequent removal. The esophagus was incised using a purse-string clamp, the traction line was ligated at its distal end, and a circular stapler was positioned at its proximal end. A 4 cm small incision was made in the middle of the upper abdomen in order to remove the tumors located in the gastric and esophageal regions. A linear cutting stapler was employed to perform the incision and closure of the gastric wall, specifically along the lesser curvature in a downward direction, parallel to the greater curvature, until reaching the second branch of the right gastric artery. A tubular stomach, about 3-4 cm wide, was made. At the same time, the esophageal tumor was resected and the lesser curvature was embedded by continuous suture. The tube stomach reached the neck incision by traction upward through the esophageal bed (Figures 4C, 4D). The posterior wall of the remnant stomach was mechanically anastomosed with the esophagus. Following the assessment of the tightness of the anastomosis and the absence of bleeding, the surgical process involved the insertion of a stomach tube and a duodenal nutritional tube. The stump was sutured utilizing a linear cutting stapler, and an absorbable suture was embedded in the anastomosis following intermittent embedding of silk suture. A drainage tube was inserted into the incision in the neck, and further suturing was performed in a layered manner for both the neck and abdomen incisions. The region and quantity of lymph nodes that were excised were delineated and thereafter submitted for pathological analysis.
In accordance with the principle of expedited rehabilitation, patients were encouraged to get out of bed early after surgery. All patients were administered nutritional support via a nasogastric tube, in addition to receiving symptomatic care including anti-inflammatory medication and electrolytes replenishment. Following the surgical procedure, the patients fasted for 2-3 weeks postoperatively. Upon discharge, a nasogastric tube was provided to facilitate nutrition. Afterwards, a re-examination was conducted after 2 weeks to extubation.
Statistical analysis
SPSS 26.0 statistical software was used to analyze the data. The measurement data were expressed as mean ± standard deviation (x ± s), and the count data were expressed as the number of cases and percentage (%). The t-test was employed to assess the differences in measurement data between the two groups, while the χ2 test was used to compare the count data. P<0.05 was considered statistically significant.
Results
Comparison of general information between the two groups of patients
Table 1 illustrated that no statistically significant changes were seen in any of the indicators between the two groups (P>0.05). There was comparability between the two groups.
| Indicators | Inflatable mediastinoscopy-assisted laparoscopic group | McKeown group | t/χ2 | P |
|---|---|---|---|---|
| (64 cases) | (56 cases) | |||
| Gender(cases) | - | - | 2.011 | 0.156 |
| Male | 38 | 26 | - | - |
| Female | 26 | 30 | - | - |
| Age[years, `x ± s (range)] | 52.19 ± 7.15 | 54.69 ± 8.31 | - | - |
| Tumor location | - | - | 1.405 | 0.495 |
| Upper thoracic | 6 | 9 | - | - |
| Mid-thoracic | 32 | 25 | - | - |
| Lower thoracic | 26 | 20 | - | - |
| Basic diseases of the chest | - | - | 2.916 | 0.088 |
| Yes | 21 | 30 | - | - |
| No | 35 | 26 | ||
| Tumor staging | - | - | 2.912 | 0.233 |
| I | 16 | 11 | - | - |
| II | 28 | 19 | - | - |
| III a | 20 | 26 | - | - |
| Tumor diameter(cm) | - | - | 2.912 | 0.233 |
| <3 | 21 | 13 | - | - |
| 03-May | 28 | 31 | - | - |
| >5 | 15 | 12 | - | - |
| Pathological type | 0.264 | 0.607 | ||
| Squamous cell carcinoma | 34 | 29 | - | - |
| Adenocarcinoma | 18 | 19 | - | - |
| Other | 12 | 8 | - | - |
| Preoperative neoadjuvant therapy | - | - | 0.146 | 0.115 |
| Yes | 20 | 16 | - | - |
| No | 44 | 40 | - | - |
Comparison of perioperative indicators between the two groups of patients
The operation time, intraoperative blood loss, 24- hour thoracic drainage volume, total drainage volume in 3 days after surgery, and length of postoperative hospital stay were shown to be significantly shorter in the group that underwent inflatable mediastinoscopy synchronized laparoscopic esophagectomy compared to the group that underwent minimally invasive McKeown surgery (P<0.05). However, no statistically significant differences were observed in terms of recurrent laryngeal nerve lymph node excision, paraesophageal lymph node excision, total lymph node excision, the time of getting out of bed for the first time after surgery, the time of eating after surgery, and the hospital stay after surgery between the two groups (P>0.05). Please refer to Table 2 for further information.
| Packet | Inflatable mediastinoscopy-assisted laparoscopic group | McKeown group | t/χ 2 | P |
|---|---|---|---|---|
| (64 cases) | (56 cases) | |||
| Operation time(min) | 134.53 ± 16.43 | 174.23 ± 29.54 | 9.244 | <0.01** |
| Intraoperative blood loss(ml) | 29.85 ± 11.71 | 106.17 ± 27.82 | 19.102 | <0.01** |
| Thoracic drainage volume 24hours postoperation (ml) | 0 | 251.84 ± 67.19 | 8.234 | <0.01** |
| Thoracic drainage volume 3 days postoperation (ml) | 102.34 ± 21.95 | 346.29 ± 31.76 | 48.271 | <0.01** |
| Time out of bed postoperation(d) | 1.34 ± 0.95 | 6.29 ± 0.76 | 31.679 | <0.01** |
| Lymph nodes para-recurrent laryngeal nerve (number) | 25.34 ± 1.25 | 19.48 ± 2.76 | 74.421 | <0.01** |
| Paraesophageal lymph nodes (number) | 31.47 ± 3.16 | 28.62 ± 2.26 | 113.685 | <0.01** |
| Total number of lymph nodes removed (number) | 54.13 ± 1.56 | 51.49 ± 1.24 | 116.971 | <0.01** |
| Postoperative hospitalization (d) | 6.68 ± 1.64 | 15.27 ± 1.76 | 113.25 | <0.01** |
| Hospitalization expenses (ten thousand) | 31571 ± 365.7 | 33596 ± 452.4 | 118 | <0.01** |
Note: The surgical time is calculated from the start of skin cutting to the completion of skin stitching; Intraoperative bleeding volume statistics: The total amount of hemostatic gauze and suction tubes; The flow rate is accurate to ml; The drainage tube time and hospitalization time are accurate to days; All postoperative complications were clinically confirmed; The total hospitalization cost is accurate to 10000 yuan.*P<0.05**P<0.01,***P<0.001.
Comparison of postoperative complications between the two groups of patients
Table 3 showed that there was no perioperative death observed in both groups. The inflatable mediastinoscopy synchronized laparoscopic esophagectomy group experienced a total of 8 complications, resulting in an overall incidence rate of 12.50%. There were a total of 8 complications in the minimally invasive McKeown group, with a total incidence of 14.29%. It was also found that the occurrence of postoperative pulmonary complications was significantly reduced in the group that underwent inflatable mediastinoscopy synchronized laparoscopic esophagectomy compared to the McKeown group (P<0.05). However, there was no statistically significant difference in the overall incidence of complications between the two groups (P>0.05).
| Packet | McKeown group | Mediastinoscope group | t/X2 | P |
|---|---|---|---|---|
| (64 cases) | (56cases) | |||
| Lung infection | 2(3.12%) | 0(0%) | - | - |
| Hoarseness | 2(3.12%) | 2(3.57%) | - | - |
| Anastomotic/tubular gastric fistula | 2(3.12%) | 1(1.79%) | - | - |
| Anastomotic stenosis | 1(1.56%) | 1(1.79%) | - | - |
| Chylothorax | 1(1.56%) | 1(1.79%) | - | - |
| Pleural effusion | 1(1.56%) | 1(1.79%) | - | - |
| Emphysema | 2(3.12%) | 0(0%) | - | - |
| Arrhythmia | 1(1.56%) | 1(1.79%) | - | - |
| Heart failure | 1(1.56%) | 1(1.79%) | - | - |
| Incision infection | 0(0%) | 0(0%) | - | - |
| Gastric emptying disorder | 1(1.56%) | 3(5.36%) | - | - |
| Total pulmonary complications | 1(1.56%) | 0(0%) | - | - |
| Overall incidence of complications | 14(21.88%) | 8(14.29%) | 4.727 | 0.030* |
Note: There was no perioperative death and common postoperative complications between the two groups.*P<0.05**P<0.01,***P<0.001.
Comparison of postoperative short-term and long-term survival rates between the two groups of patients
| Packet | McKeown group | Mediastinoscope group | t/X2 | P |
|---|---|---|---|---|
| (64 cases) | (56cases) | |||
| 30 days postoperation | 64(100%) | 56(100%) | 0.094 | 0.9336 |
| 3 months postoperation | 64(100%) | 56(100%) | 0.094 | 0.9336 |
| 6 months postoperation | 62 (96.88%) | 53 (94.64%) | 0.373 | 0.542 |
| 1year postoperation | 59 (92.19%) | 50(89.29%) | 0.302 | 0.583 |
| 3year postoperation | 50 (78.13%) | 43(76.79%) | 0.031 | 0.861 |
| 5year postoperation | 39 (60.94%) | 36(60.71%) | 0.143 | 0.705 |
Note: There was no significant difference between the two groups at 30 days postoperation, 3 months postoperation, 6 months postoperation, 1 year postoperation, 3 year postoperation and 5year postoperation.
There were no statistically significant differences observed in the overall survival rates between the two groups at 30 days, 3 months, 6 months, 1 year, and 3 years post-surgery (P>0.05). There was a statistically significant difference in the 5-year overall survival rate between the two groups (P<0.05), see Table 4.
Discussion
Esophageal cancer is one of the malignant tumors treated by thoracic surgery [5]. However, surgical treatment of esophageal cancer is traumatic. This is mostly due to the deep anatomical location of esophageal cancer, which is in close proximity to vital organs like the aorta and trachea. Therefore, intraoperative risk is high [6].
The advantages associated with inflatable mediastinoscopy synchronized laparoscopic esophagectomy is its avoidance of entry into the pleural cavity. This characteristic allows for the prevention of compression on pulmonary tissue and the displacement of surgical instruments, which are common occurrences in traditional thoracoscopic surgery [7]. However, at present, surgery for inflatable mediastinoscopy synchronized laparoscopic esophagectomy lacks popularity both domestically and internationally, with just a decade of use observed even in prominent local medical institutions. With effective collaboration between the Department of Anesthesiology and the surgical operating team, the surgical team employs mediastinoscopy to dissect the esophagus via the neck, and laparoscopy to dissect the stomach and lower esophagus through the abdomen. Ultimately, the mediastinoscopy and laparoscopy procedures will be integrated in the thoracic region, resulting in the full removal of the esophagus. Additionally, a cervical anastomosis was performed [8].
Our surgical team believes that the utilization of inflatable mediastinoscopy synchronized aparoscopic esophagectomy not only results in a notable reduction in operation time, but also facilitates lymph node excision with comparable efficacy to the widely accepted McKeown approach. This surgical approach enables surgeons to achieve a learning curve of over 50 cases. It is particularly applicable to patients with esophageal cancer who have impaired lung function, significant tuberculosis-related alterations in the thoracic cavity, and extensive thoracic adhesions. By opting for surgical treatment, these patients can potentially attain a longer survival period.
Our team proposed that mediastinoscope and laparoscope are performed simultaneously. During the operation, the patient’s position does not need to be changed, and the patient only needs to rotate the neck around and around to cooperate with the operation, which significantly reduces the total operation time (generally completed within 2.5-3.0 hours). The simultaneous execution of operations by two groups of surgeons and two groups of instrument nurses resulted in several benefits. These included a reduction in anesthesia time and intraoperative blood loss, as well as a decrease in the waiting time for patient postural changes and re-disinfection of towels. Therefore, this technique significantly shortened the overall operation time, the patient’s surgical trauma time, and the occurrence of postoperative complications. In addition, the absence of a thoracic incision and thoracic vascular damage resulted in little surgical bleeding and a reduction in the duration of the procedure due to decreased need for repeated hemostasis. Furthermore, the utilization of this technique in current clinical practice extended the surgical indications of the widely accepted McKeown procedure for the comprehensive treatment of esophageal cancer. This was due to its ability to avoid the need for single-lung ventilation, without damaging the intercostal nerves, and provide notable benefits in terms of patient comfort and expedited postoperative recovery. Hence, our surgical team maintained that the selection criteria for surgical patients should encompass the following indications.
1) early-stage esophageal cancer (stage 0 and stage I); 2) patients diagnosed with upper esophageal cancer exhibiting a lesion length measuring less than 3.0 cm, middle esophageal cancer with a lesion length less than 5.0 cm, and lower esophageal cancer with a lesion length less than 7.0 cm, all falling under stage II and III of esophageal cancer, and displaying no evident external invasion of the tumor; 3) some patients diagnosed with stage III esophageal cancer, presenting preoperative external invasion, who demonstrated favorable health status subsequent to preoperative induction radiotherapy. The contraindications for surgery should encompass the following criteria.
1) The cancer exhibits extensive invasion or demon- strates evident external infiltration, or its removal becomes unfeasible due to invasion of nearby vital organs. 2) The esophagus presents symptoms of penetration or distant metastasis. 3) Patients with serious heart, lung, liver, and kidney dysfunction cannot tolerate surgery. In addition, patients with severe cachexia are also unable to undergo surgery.
Two transverse neck incisions effectively excised 101 or 104 bilateral lymph nodes. The surgical procedure relied upon the exposure and dissection of the left recurrent laryngeal nerve via the incision made on the left side of the neck. It is advised to exercise caution when dissecting and exposing the left recurrent laryngeal nerve, as excessive manipulation might lead to demyelination damage of the nerve and subsequent postoperative hoarseness. The utilization of specialized upper and lower mediastinal retractors was imperative for conducting dissections within narrow anatomical regions. Maryland forceps were used to separate and excise tissues during operation, which generate minimal smoke during thermal conduction, hence preserving the visual clarity of the surgical field. The inflation pressure of artificial mediastinal emphysema was controlled at 10-12 mmHg, and the abdominal inflation pressure was controlled at 12-14 mmHg [9]. It not only facilitated the visualization of the surgical region, but also mitigated the risk of significant CO2 accumulation [10].
The creation of an artificial environment for mediastinal inflation resulted in several benefits, including improved eyesight, enlarged operating area, and enhanced visibility of the intact exposed trachea, thoracic duct, and other structures [11].
The utilization of inflatable mediastinoscopy demonstrated efficacy in overcoming challenges associated with a constricted mediastinal area, restricted surgical maneuverability, and intricate lymph node excision. At the same time, it completely removed the surrounding lymph nodes, including-mediastinal lymph nodes, recurrent laryngeal nerve lymph nodes, subcarinal lymph nodes, periesophageal lymph nodes, diaphragm and abdominal lymph nodes above the upper edge of the pancreas. Currently, there is a significant debate about the completion of regional lymph node dissection in the context of inflatable mediastinoscopy synchronized laparoscopic esophagectomy. The number of lymph nodes excised in the middle and upper mediastinum is few, especially the lymph nodes along the right recurrent laryngeal nerve [12]. Our team suggested the utilization of inflated mediastinoscopy synchronized laparoscopic esophagectomy, employing a pair of transverse incisions in the neck. Under direct visualization, it was possible to perform dissection of the lymph nodes located along the right recurrent laryngeal nerve. Additionally, the lymph nodes situated beneath the aortic arch, namely those adjacent to the left tracheobronchial region, could be visualized in a more unobstructed vertical manner, devoid of interference from the aortic arch. Furthermore, it had the potential to successfully perform a thorough excision of the left paratracheobronchial lymph nodes (No.106tbL group), which are typically considered inoperable, with a single incision on the left side. The right cervical approach enabled the execution of a comprehensive excision of the right middle and upper mediastinal lymph nodes under direct visualization. This technique offered a wide visual field, facilitating the dissection of para-esophageal and bilateral recurrent laryngeal nerve lymph nodes with relative ease. One notable advantage of employing this method was its ability to avoid the need for thoracotomy, hence preserving the structural integrity of the thoracic cavity. Additionally, this strategy entailed reduced surgical trauma, diminished pain, and expedited postoperative recuperation. After surgeons successfully completing the learning curve of 50 cases, the surgeon could completely achieve the same effect as the mainstream McKeown’s approach. In certain cases, inflatable mediastinoscopy synchronized laparoscopic esophagectomy may be considered as an alternative treatment option for patients with esophageal cancer, particularly those who have cardiopulmonary dysfunction and for whom the conventional McKeown technique is not appropriate.
In summary, the technique of inflatable mediastinoscopy synchronized laparoscopic esophagectomy involved the creation of a mediastinal tunnel to complete the procedure. Additionally, a tubular stomach was constructed through a minor incision in the upper abdomen, followed by an anastomosis in the left neck. Notably, this approach has been found to be associated with a minimal risk of chest complications. Therefore, the proposed surgical procedure can serve as a supplementary procedure to the existing McKeown esophagectomy technique, and can be effectively implemented in clinical practice.
Conclusion
Our surgical team believes that the inflatable mediastinoscopy synchronous laparoscopic radical resection of esophageal cancer not only greatly shortens the surgical time during the process, but also achieves homogenized therapeutic effects with mainstream McKeown procedures through lymph node dissection. Its advantages lie in avoiding thoracotomy and maintaining the integrity of the chest, as well as its minimal surgical trauma, mild patient pain, and fast postoperative recovery. The inflatable mediastinoscopy synchronous laparoscopic surgery for esophageal tumors and lymph node dissection is completed in the mediastinal tunnel, and the tubular stomach is made through a small incision in the upper abdomen, with left neck anastomosis and no chest complications. It can be used as a new supplementary surgery to the mainstream McKeown surgery for esophageal cancer, and actively promoted and applied in clinical practice.
Declaration
Ethics approval
Henan University Biomedical Research Ethics Committee (HUSOM2023-348).
Consent for publication
All authors have given final approval of the version and agreed with the publication of this study here.
Author contributions
Hai-Tao Wei was the main author of the manuscript, and all the other authors were involved in the operation, data collection and data analysis. All authors have read and agreed to publish the final manuscript and agree to be responsible for all aspects of the study.
Funding
This work was supported by the Science and Technology Department of Henan, Grant number: (No.242102310100;No.242102310280) China.
Acknowledgments
We thank all the survey respondents who participated in the study.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest statement
The authors declare no conflict of interest.
References
- Zhang L, Li L, Chen X, Yuan S, Xu T, Zhao W, et al. Evodiamine inhibits ESCC by inducing M-phase cell-cycle arrest via CUL4A/p53/p21 axis and activating noxa-dependent intrinsic and DR4-dependent extrinsic apoptosis. Phytomedicine 2023;108:154493.
[Crossref][Google Scholar] [Pubmed]
- Zhang Y, Zhang Y, Peng L, Zhang L. Research Progress on the predicting factors and coping strategies for postoperative recurrence of esophageal cancer. Cells 2022;12(1):114.
[Crossref] [Google Scholar] [Pubmed]
- Huang S, Yang T, Wang W, Huang G, Chen B, Chen P, et al. Thoracoscopic radical esophagectomy combined with left inferior pulmonary ligament lymphadenectomy for esophageal carcinoma via the right thoracic approach: A single-center retrospective study of 30 cases. Medicine 2021 11;100(23):e26302.
[Crossref] [Google Scholar] [Pubmed]
- Casas MA, Angeramo CA, Harriott CB, Schlottmann F. Surgical outcomes after totally minimally invasive Ivor Lewis esophagectomy. A systematic review and meta-analysis. Eur J Surg Oncol 2022;48(3):473-481.
[Crossref] [Google Scholar] [Pubmed]
- Ahmed N, Owen J, Abdalmassih M, Khan J, Nugent Z, Qing G, et al. Outcome of locally advanced esophageal cancer patients treated with perioperative chemotherapy and chemoradiotherapy followed by surgery. Am J Clin Oncol 2021;44(1):10-17.
[Crossref] [Google Scholar] [Pubmed]
- Wang Y, Ye D, Kang M, Zhu L, Pan S, Wang F, et al. Risk factors and patterns of abdominal lymph node recurrence after radical surgery for locally advanced thoracic esophageal squamous cell cancer. Cancer Manag Res 2020:3959-3969.
[Crossref] [Google Scholar] [Pubmed]
- Liu C, Chen Z, Wei R, Huang K, Wu B, Xu Z, et al. Intra-operative events and countermeasures during esophagectomy via transcervical incision inflatable single-port mediastinoscope combined with laparoscopy. J Thorac Dis 2021;13(1):133-139.
[Crossref] [Google Scholar] [Pubmed]
- Huang ZN, Liu CQ, Guo MF, Xu MQ, Sun XH, Wang GX, et al. Clinical analysis of inflatable video-assisted mediastinoscopic transhiatal esophagectomy combined with laparoscopy. Zhonghua Wai Ke Za Zhi 2023;61(1):48-53.
[Crossref] [Google Scholar] [Pubmed]
- Yibulayin X, Xu K, Yibulayin W, Abulaiti A, Wu Z, He D, et al. Single-port inflatable mediastinoscopic esophagectomy is a cure for esophageal cancer patients: Case report. Medicine 2022;101(46):e31619.
[Crossref] [Google Scholar] [Pubmed]
- Wang G, Sun X, Li T, Xu M, Guo M, Liu C, et al. Study of the short-term quality of life of patients with esophageal cancer after inflatable videoassisted mediastinoscopic transhiatal esophagectomy. Fron Surg 2023;9:981576.
[Crossref] [Google Scholar] [Pubmed]
- Yin Q, Liu H, Song Y, Zhou S, Yang G, Wang W, et al. Clinical application and observation of single-port inflatable mediastinoscopy combined with laparoscopy for radical esophagectomy in esophageal squamous cell carcinoma. J Cardiothorac Surg 2020;15:1-7.
[Crossref][Google Scholar] [Pubmed]
- Wang X, Li X, Huo W, Cheng H, Zhang B, Zhong H, et al. The procedure of single-port inflatable mediastinoscopy and laparoscopic surgery for radical esophagectomy. Mediastinum 2019;3:22.
[Crossref] [Google Scholar] [Pubmed]