Research Article - Archives of Clinical and Experimental Surgery (2023)
Post Sleeve Gastrectomy Fistula: Experimental Model in Pigs
Giuliana Fulco Gonçalves1, Italo Barros Miranda1, Amália Cinthia Meneses do Rêgo2, Francisco Irochima Pinheiro2 and Irami Araújo-Filho3*2Department of Biotechnology, Potiguar University, Natal, Brazil
3Department of Surgery, Federal University of Rio Grande do Norte, Natal, Brazil
Irami Araújo-Filho, Department of Surgery, Federal University of Rio Grande do Norte, Natal, Brazil, Email: irami.filho@uol.com.br
Received: 10-Jun-2023, Manuscript No. EJMACES-23-102049; Editor assigned: 12-Jun-2023, Pre QC No. EJMACES-23-102049 (PQ); Reviewed: 27-Jun-2023, QC No. EJMACES-23-102049; Revised: 04-Jul-2023, Manuscript No. EJMACES-23-102049 (R); Published: 11-Jul-2023
Abstract
Introduction: Bariatric surgery is considered the primary treatment against severe obesity, as it is effective in sustained weight loss and reduces the prevalence of associated comorbidities. However, bariatric surgery is not free of complications observed at various postoperative stages, including fistulas.
Purpose: To describe the creation of an experimental model of a gastric fistula after the performance of Sleeve Gastrectomy (SG) in pigs.
Methods: Sleeve gastrectomy was performed in pigs, using tubes for postoperative fistula induction, following the ethical principles of animal experimentation.
Results: After an observation period of 21 days, it was possible to confirm the formation of the fistulous tract. None of the animals died.
Conclusion: The experimental model of post-Sleeve Gastrectomy fistula in pigs is efficient, effective, and reproducible and can be used in future studies to test new diagnostic methods and innovations in the therapeutic management of postoperative fistulas.
Keywords
Bariatric surgery; Gastrectomy; Postoperative complications; Fistula; Experimental animal model; Swine
Introduction
Morbid obesity is a chronic disease considered by the World Health Organization (WHO) as a worldwide epidemic related to excess body fat, dietary profile, physical activity, and population genetics [1]. It is estimated that by 2025, 2.3 billion adults worldwide will be overweight, of which 700 million individuals will be obese, with a Body Mass Index (BMI) above 30 and severe obesity from 35 [1-3].
Excess weight is associated with diabetes, hypertension, cardiac and pulmonary dysfunction, deep vein thrombosis, depression, predisposes patients to infections, incisional hernias, and increases operative morbidity and mortality, related to complex surgical risks, intraoperative technical difficulties, and serious surgical complications [2-4].
Diet therapy is the first option in the fight against overweight associated with a change in life habits, such as adopting daily physical exercises for a balanced and healthy weight loss. However, studies have shown that, on average, 75%-95% of obese people fail to maintain weight loss diets due to the strong relationship between overweight and binge eating [5,6].
Thus, bariatric surgery is considered the primary treatment against severe obesity, as it is effective in sustained weight loss and reduces the prevalence of associated comorbidities [7]. Concurrent with the high prevalence of obesity, bariatric surgery has increased in number and different types of techniques. It is currently one of the most performed elective surgeries in the world [8,9].
The most common bariatric operations are Laparoscopic Sleeve Gastrectomy (LSG), Roux-en-Y Gastric Bypass (RYGB), and Adjustable Gastric Band (AGB). The Biliopancreatic Diversion is also with Duodenal Switch (BPD-DS) and Intragastric Balloon (IB) [10-12]. In 2018, it was estimated that 63,969 and 252,000 bariatric procedures were performed in Brazil and the United States, respectively, with emphasis on Laparoscopic Sleeve Gastrectomy, among the various techniques used [13,14].
Sleeve Gastrectomy reduces gastric volume and appetite by lowering ghrelin levels and increasing GLP-1 production, contributing to weight loss. In this sense, Laparoscopic Sleeve Gastrectomy (LSG) has become a standard procedure for the surgical treatment of patients with different degrees of obesity. Advantages include no bowel bypass, reduced risk of internal hernia obstruction, and sustained weight loss [15].
Despite the benefits above, bariatric surgery is not free of complications observed at various postoperative stages, depending on the procedure used. The mechanisms involved are associated with the surgical technique, the surgical team’s experience, ischemia, use of medications, and the presence of comorbidities [16]. The management of these complications has evolved due to technological advances to obtain the best results, where early diagnosis and treatment are important prognostic factors [6,17].
Regarding sleeve gastrectomy, there is a risk of staple line bleeding, strictures (the mid or distal portion of the residual stomach), reflux, and gastric fistulas that increase morbidity and mortality, resulting in abdominal sepsis multiple organ failure, and death [1,7-9].
Gastric fistula is the most feared complication of GS due to its complex management and long clinical course. Up to 90% of post-SG fistulas occur at the Esopha Gogastric Junction (EGJ) and rarely at the distal part of the staple line. The prevalence of fistulas is 2.2%–2.4%, according to systematic literature reviews [3,9].
In Roux-en-Y gastroplasty, fistulas are more frequent, with an incidence between 0.3% and 8.3%, especially when the stomach is not separated from the excluded gastric pouch [18]. Since bariatric surgery represents a challenge to the body due to the endocrine and metabolic response, each technique must be tested and perfected beforehand to minimize any possible intercurrence [3,5-7].
Based on the above, the feasibility of the procedure and its results, complications, and forms of treatment, in particular postoperative fistulas, can be previously observed through the adoption of experimental models. In this way, the chosen animals must be physiologically like humans, guarantee technical equivalence and reproducibility [4,8].
The objective of the present study was to describe the creation of an experimental model of a gastric fistula after the performance of Sleeve Gastrectomy (SG) in pigs to provide an alternative for future studies involving different studies types of treatment in obese patients with this surgical complication.
Materials and Methods
Experimental design
The Ethics Committee approved the experimental protocol on the Use of Animals, Potiguar University (UnP), number 3251/2019, Brazil. Animals were handled following the Guide for the Care and Use of Laboratory Animals, US National Research Council, 1996. The Institutional Committee on Ethics in the Use of Animals approved the research project under the protocol. Brazilian College of Animal Experimentation (Law 11.794/2008-CONCEA– Brazilian College of Animal Experimentation). Three crossbred (Large white × Landrace) male pigs, 25.4 ± 1.8 kg (73 ± five days of age), were purchased from a commercial farm and housed in the Animal Facility facilities of UnP one week before the surgery for acclimatization. The surgeries were performed in the experimentation sector of the Animal House at UnP, where the animals were randomly separated and placed in individual pens lined with wood shavings. During the first week, the animals were weighed (Electronic Scale for Pigs/Pig/Filizola®2022), identified with an ear tag, dewormed (1% Ivermectin, 1 mL/35 kg/SC, Crack®, Chile), injected with Vitamin ADE, 0.5 mL/animal IM, Drag®Pharma), and vaccinated against erysipelas (2 mL/animal) and mycoplasma (2 mL/animal/SC, Intervet Veterinaria®, Chile); for adaptation, remaining one week in the acclimatization period, under a standard controlled temperature of 20°C ± 22°C, humidity around 45% ± 15% and ambient lighting, with an inverted 12-hour light-dark cycle. In addition, the environment remained under noise below 60 dB, and the diet offered was standardized with commercial food from Purina® and filtered water ad libitum. The animals were evaluated daily for weight and performance status. There was a random division, following the principles of the 3R’s-Reduce, Refine and Replace-to guarantee the bioethical principles of the study. The three animals underwent the same operative procedure and were observed for 21 days, a period sufficient for the emergence and maturation of the spent cutaneous fistula, after removing the tubes.
Anesthetic procedure/ preoperative preparation
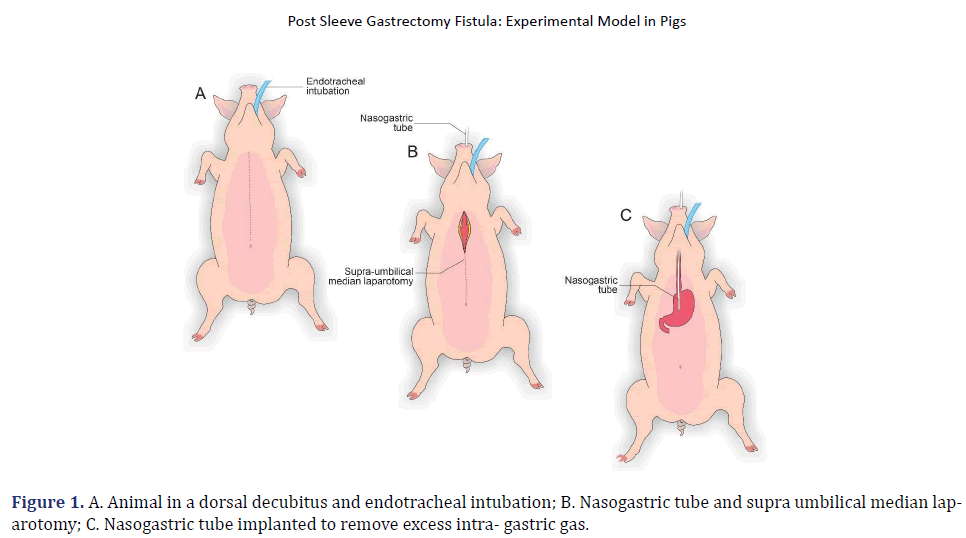
The pigs were submitted to 12 h of fasting. They were sedated during the preoperative period with an intramuscular association of 2 mg/kg of acepromazine (Acepran ®1%) and 0.1 mg/kg of midazolam (Dormire®). After establishing intravenous fluid therapy, anesthesia was induced with 1 mg/kg of sodium thiopental (Thiopentax®/Cristália) and 10 mg/mL propofol (Provive®1%). After endotracheal intubation, the anesthesia was maintained with Isoflurane (Isoforine®) and oxygen under mechanical ventilation. The animals were placed in a dorsal decubitus, and a trichotomy of the entire ventral abdominal region was performed by surgical antisepsis with 0.5% Chlorhexidine Gluconate (Vic Pharma®). The animals were placed on a surgical table with a heated mattress, and the surgery began (Figure 1).
Surgical procedure
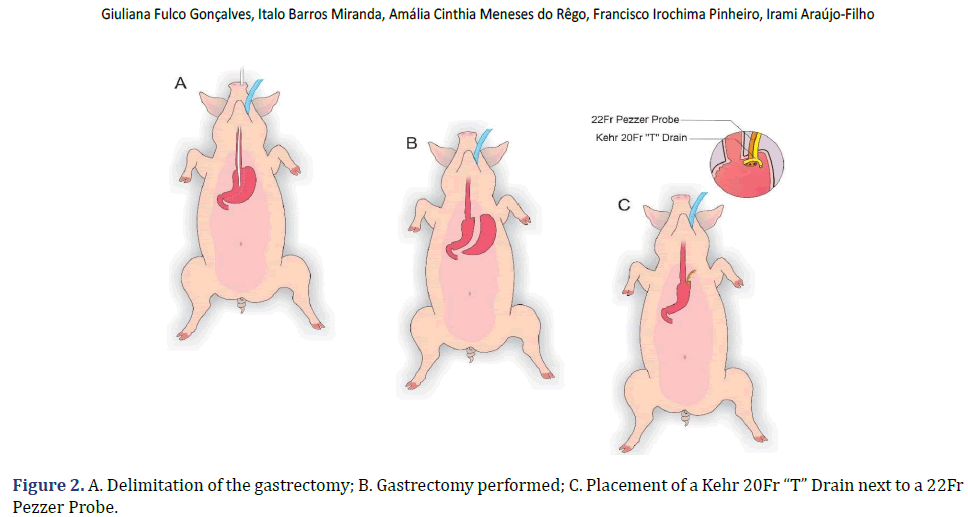
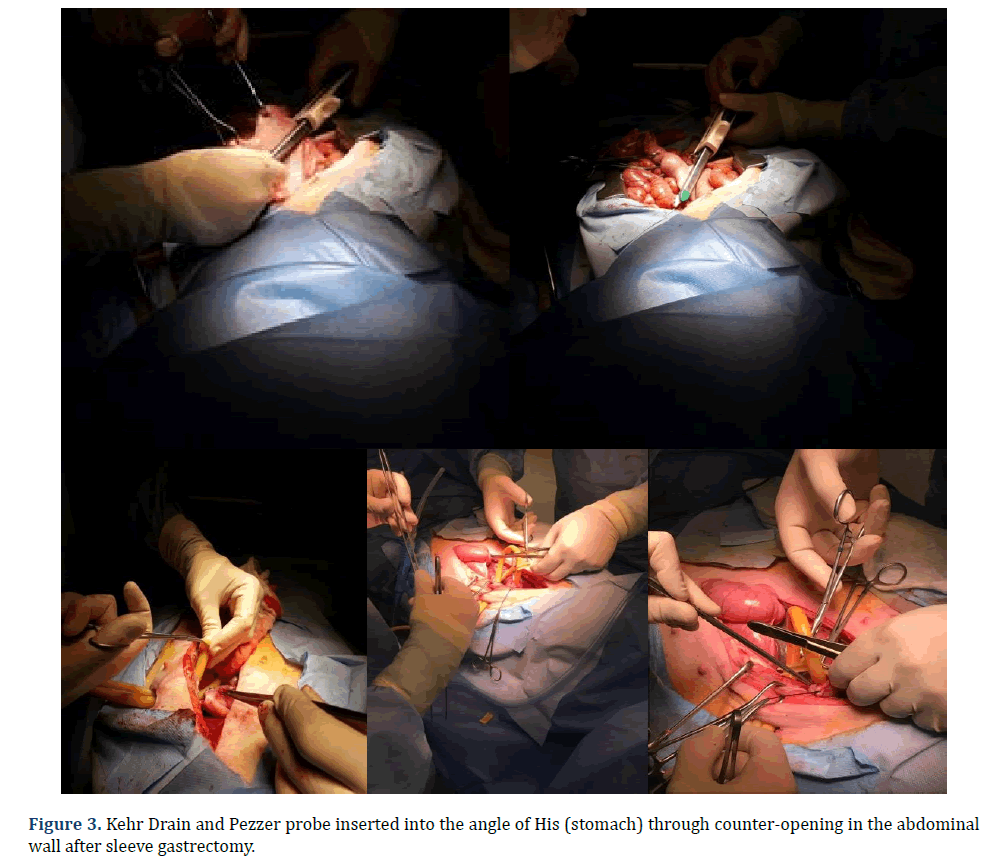
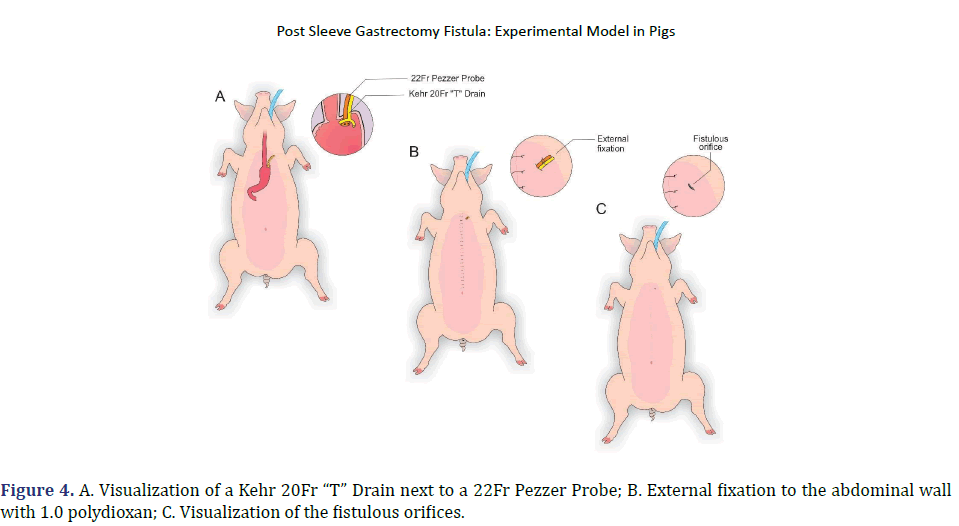
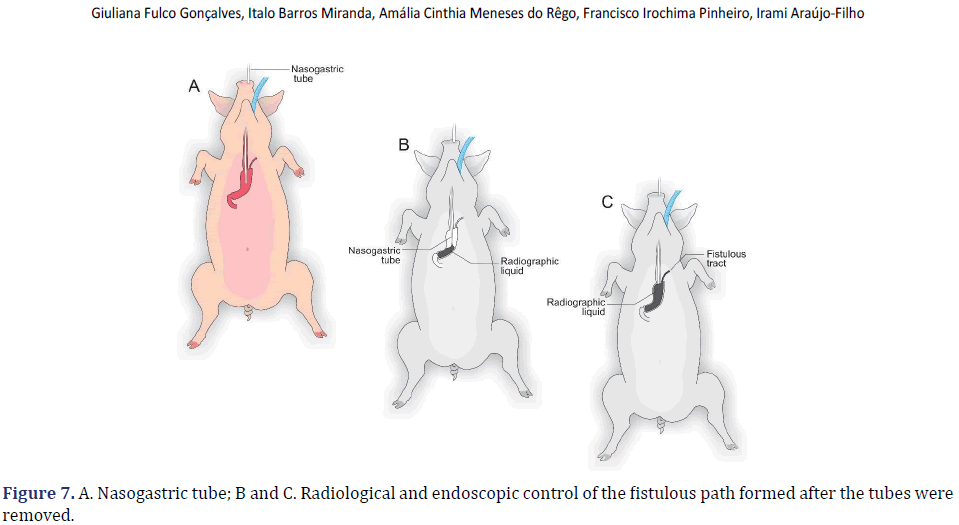
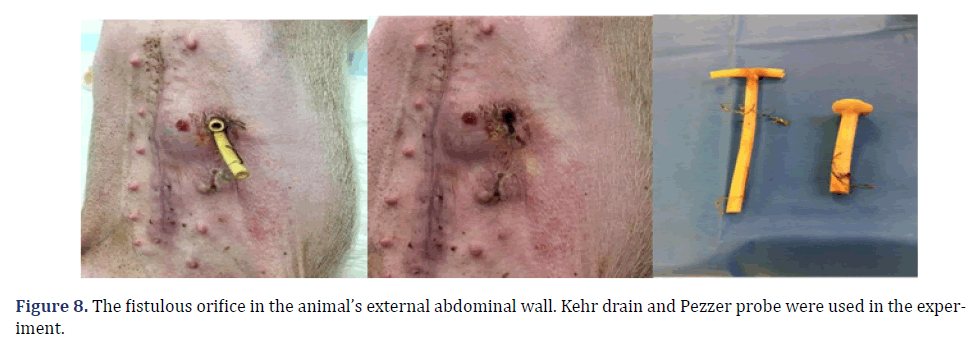
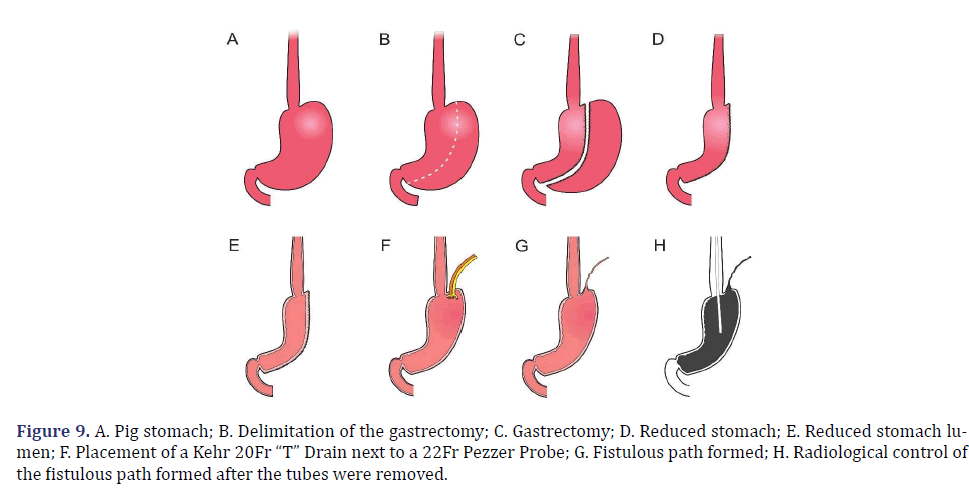
In each animal, the same surgical procedure was applied. A supra-umbilical median laparotomy was performed, opening the animal’s abdominal cavity in planes (pig) and placing cavity retractors to expand the operative field. A nasogastric tube was implanted to remove excess intra-gastric gas. The stomach was isolated while the short vessels between the stomach and spleen were ligated and sectioned to allow sleeve gastrectomy without bleeding. Starting with the gastric antrum, covering the entire greater curvature of the stomach, sleeve gastrectomy was performed through stapling and sectioning of the greater curvature starting from the antrum and going to the angle of His, using 3-4 Selectable NTLC75-Linear Cutter with 3D Staples (ETHICON-Johnson&Johnson®). After this, the stomach was reduced to 30%-40% of its initial volume. Then, a gastrostomy was performed at the angle of His with the placement of a Kehr 20Fr “T” Drain next to a 22Fr Pezzer Probe, which was inserted into the stomach through a counter-opening in the pig’s abdominal wall. The gastrostomy was internally fixed to the abdominal wall, parietal peritoneum, using 2.0 propylene thread (ETHICON-Johnson&Johnson®). The Kehr drain and the Pezzer probe were externally fixed to the abdominal wall with 1.0 polydioxanone thread (ETHICON-Johnson& Johnson®), followed by the creation of a subcutaneous tunnel for gastric secretion drainage to ensure that the devices couldn’t be removed by the animal itself in the postoperative period. At the end of the operation, the abdominal wall was closed in layers. To avoid hypothermia and consequent acidosis and postoperative hemorrhages, thermal mattresses were used on the operating table and heater and cage seals during the transport of the operated animal back to the vivarium. Postoperative pain control was performed through the administration of Meperidine (solution) 50 mg/2 mL–Intramuscularly (IM-DOLOSAL® -Cristália), 50- 100 mg/day, the dose was repeated every 3 h to 4 h. After anesthetic recovery (12 ± 2 h after surgery), the animals were kept in individual pens, lined with wood shavings, drinkers and feeders. The ambient temperature was maintained between 22 and 26°C using heaters (Figures 2-5).
Observational/fistulous period
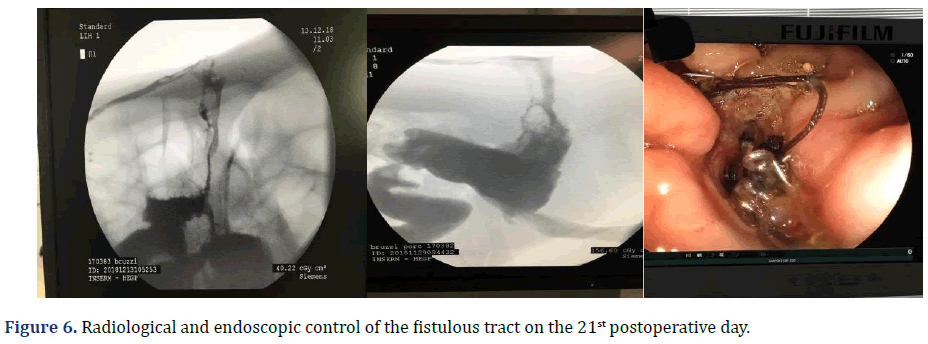
The total observation period of the study was 21 days. The animals received a soft liquid diet while observed for vomiting, pain, or lack of appetite in the first three postoperative days. Then, the regular diet from Purina ® and filtered water ad libitum was resumed for the remainder of the study. On the second postoperative day, it was possible to observe the output of gastric secretion through the tubes placed in the stomach. None of the three animals died. A weight-loss varied in each animal, around 15-25% of the initial weight. On the 21st postoperative day, the animals were again anesthetized. Then the radiological and endoscopic control of the fistulous path formed before and after the tubes were removed and the direct visualization of the fistulous orifices in the abdominal wall of the three animals. After verifying that there was actually a fistulous tract formed, with gastric secretion output by the various methods of analysis, the animals still anesthetized were killed without suffering after intracardiac transthoracic injection of an anesthetic overdose of Sodium Thiopental 5 mg/kg (Thiopentax®-Cristália) using an epidural needle guided by a portable echocardiogram device (HM70®-Samsung)–(Figures 6-9).
Results and Discussion
Postoperative complications will always be a reality in the clinical practice of surgeons. Fistulas are a permanent challenge, as they can progress to abscesses, peritonitis, septic shock, and death of affected patients [12-14].Regarding morbidly obese patients undergoing bariatric surgery, the appearance of the postoperative fistula is added to hypertension, diabetes, and other comorbidities present in these patients, greatly increasing morbidity and mortality [19-20]. Understanding the mechanisms involved in the appearance of postoperative fistulas, risk factors, and the diagnosis and knowledge of early forms of treatment are paramount to increasing survival in obese patients with fistulas when it comes to bariatric surgery [15-17]. In this sense, the experimental model demonstrated in the present study presents itself as a viable and scientifically validated alternative to improve the therapeutic management of fistulas after Sleeve Gastrectomy [21-22].
We chose to use pigs because they are animals like humans in terms of anatomy, genetics, and physiology and, therefore, serve the purpose of replacing them in clinical tests, mainly in the application of new techniques and therapeutic modalities, until reaching the desired safety and efficacy, before being applied in daily clinical practice [3,4,8-10]. Another important aspect is that the present experimental model of fistula after Sleeve Gastrectomy is similar to what occurs in daily clinical practice, where fistulas arise more frequently at the Angle of His (esophageal-gastric junction)[23].
Added to this is that it is a safe experimental model, easy to perform, low cost, reduced morbidity and mortality, present weight loss, and requires little observation time regarding the formation of the postoperative fistulous path. In addition, to reproduce in a very similar way to what occurs in humans, a fact proven through fistulography and endoscopic control [15,20-22].
Currently, studies demonstrate several strategies in fistula treatment after Sleeve Gastrectomy. Such research ranges from the use of stem cells, the use of self-expanding stents, interventional radiology, the transformation of Sleeve Gastrectomy into a Roux-Y-shaped bypass, addition to the most used which is the endoscopic approach with the use of catheters to direct the fistula, suction mechanisms, even performing total gastrectomy in cases considered to be of high severity, among others [14,23-25]. It is important to emphasize that the study has limitations, as there was not enough time to complete other stages due to the pandemic caused by COVID-19, which forced the suspension of the different stages of the research, such as the performance of the procedure by video laparoscopy in addition to the biophysical chemical analyzes proposed in the initial project, since the experiments started in November 2019 with an estimated time of duration until June 2020.
However, we believe that we have collaborated in some way to disseminate our preliminary results since we were at risk of losing data and, therefore, of the opportunity to contribute, through the publication of our results, to the dissemination of scientific knowledge.
Conclusion
In conclusion, based on the methodology used, the experimental model of post-Sleeve Gastrectomy fistula is efficient, effective, and reproducible and can be used in future studies to test new diagnostic methods and, above all, innovations in the therapeutic management of postoperative fistulas of the obese patients undergoing mesh surgical technique.
Acknowledgments
The authors thank the Federal University of Rio Grande do Norte, Potiguar University, and Liga Contra o Cancer for supporting this study.
Funding
Pro-Rectory of Postgraduate Studies-Federal University of Rio Grande do Norte-Natal-Brazil.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Estupinam O, Oliveira AL, Antunes F, Galvão M, Phillips H, Scheffer JP, et al. Technical innovation: Intragastric Single Port Sleeve Gastrectomy (IGSG). A feasibility survival study on porcine model. Acta Cir Bras 2018;33:95-101.
[Crossref] [Google Scholar] [Pubmed]
- Park JY. Diagnosis and management of postoperative complications after sleeve gastrectomy. J Metab Bariatr Surg 2022;11(1):1-12.
[Crossref] [Google Scholar] [Pubmed]
- Stefura T, Kacprzyk A, Droś J, Chłopaś K, Wysocki M, Rzepa A, et al. The hundred most frequently cited studies on sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne 2020;15(2):249-267.
[Crossref] [Google Scholar] [Pubmed]
- Frohman HA, Rychahou PG, Li J, Gan T, Evers BM. Development of murine bariatric surgery models: lessons learned. J Surg Res 2018;229:302-310.
[Crossref] [Google Scholar] [Pubmed]
- Arble DM, Evers SS, Bozadjieva N, Frikke-Schmidt H, Myronovych A, Lewis A, et al. Metabolic comparison of one-anastomosis gastric bypass, single-anastomosis duodenal-switch, Roux-en-Y gastric bypass, and vertical sleeve gastrectomy in rat. Surg Obes Relat Dis 2018;14(12):1857-1867.
[Crossref] [Google Scholar] [Pubmed]
- Stevenson M, Lee J, Lau RG, Brathwaite CE, Ragolia L. Surgical mouse models of vertical sleeve gastrectomy and Roux-en Y gastric bypass: A review. Obes Surg 2019;29:4084-4094.
[Crossref] [Google Scholar] [Pubmed]
- Marie L, Masson C, Gaborit B, Berdah SV, Bège T. An experimental study of intraluminal hyperpressure reproducing a gastric leak following a sleeve gastrectomy. Obes Surg 2019;29:2773-2780.
[Crossref] [Google Scholar] [Pubmed]
- Rebibo L, Dhahri A, Maréchal V, Fumery M, Delcenserie R, Regimbeau JM, et al. Gastric leaks after sleeve gastrectomy: No impact on weight loss, co-morbidities, and satisfaction rates. Surg Obes Relat Dis 2016;12(3):502-510.
[Crossref] [Google Scholar] [Pubmed]
- Delko T, Hoffmann H, Kraljević M, Droeser RA, Rothwell L, Oertli D, et al. Intraoperative Patterns of Gastric Microperfusion During Laparoscopic Sleeve Gastrectomy. Obes Surg 2017;27(4):926-932.
[Crossref] [Google Scholar] [Pubmed]
- Ozdenkaya Y, Olmuscelik O, Basim P, Saka B, Arslan NC. The effect of fibrin glue in preventing staple-line leak after sleeve gastrectomy. An experimental study in rats. Acta Cir Bras 2019;34(8): e201900801.
[Crossref] [Google Scholar] [Pubmed]
- Varban OA, Sheetz KH, Cassidy RB, Stricklen A, Carlin AM, Dimick JB, et al. Evaluating the effect of operative technique on leaks after laparoscopic sleeve gastrectomy: A case-control study. Surg Obes Relat Dis 2017;13(4):560-567.
[Crossref] [Google Scholar] [Pubmed]
- Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: Review of its prevention and management. World J Gastroenterol 2014;14;20(38):13904-13910.
[Crossref] [Google Scholar] [Pubmed]
- van Wezenbeek MR, van Oudheusden TR, de Zoete JP, Smulders JF, Nienhuijs SW. Conversion to Gastric Bypass After Either Failed Gastric Band or Failed Sleeve Gastrectomy. Obes Surg 2017;27(1):83-89.
[Crossref] [Google Scholar] [Pubmed]
- Iannelli A, Debs T, Martini F, Benichou B, Ben Amor I, Gugenheim J, et al. Laparoscopic conversion of sleeve gastrectomy to Roux-en-Y gastric bypass: indications and preliminary results. Surg Obes Relat Dis 2016;12(8):1533-1538.
[Crossref] [Google Scholar] [Pubmed]
- Alharbi SR. Plain X-ray findings of post sleeve gastrectomy gastric leak. J Clin Imaging Sci 2022;12:28.
[Crossref] [Google Scholar] [Pubmed]
- Benois M, Lecorgne E, Kassir R, Piche M, Amor VB, Chenaitia H, et al. Mesenchymal Stem Cells and PRP Therapy Favorize Leak Closure After Sleeve Gastrectomy in Zucker Rats. Obes Surg 2022;32(4):1251-1260.
[Crossref] [Google Scholar] [Pubmed]
- Fabian T, Robinson T, Naile L, Smith MP, McErlean M. Blind nasogastric tube advancement following sleeve gastrectomy: An animal model. Surg Endosc 2020;34(1):257-260.
[Crossref] [Google Scholar] [Pubmed]
- Laura M, Mylene L, Christophe B, Boris H, Christophe M, Konstantinos A, et al. Establishing a Reproducible Murine Animal Model of Single Anastomosis Duodenoileal Bypass with Sleeve Gastrectomy (SADl-S). Obes Surg 2018;28(7):2122-2125.
[Crossref] [Google Scholar] [Pubmed]
- Ueda K, Gagner M, Milone L, Bardaro SJ, Gong K. Sleeve gastrectomy with wrapping using polytetrafluoroethylene to prevent gastric enlargement in a porcine model. Surg Obes Relat Dis 2008;4(2):84-90.
[Crossref] [Google Scholar] [Pubmed]
- Boza C, Gagner M, Devaud N, Escalona A, Muñoz R, Gandarillas M, et al. Laparoscopic sleeve gastrectomy with ileal transposition (SGIT): A new surgical procedure as effective as gastric bypass for weight control in a porcine model. Surg Endosc 2008;22(4):1029-1034.
[Crossref] [Google Scholar] [Pubmed]
- Weng SG. Modified biliopancreatic diversion for GK rats: A proposal for a simpler technique and mechanism research. Obes Surg 2012;22(6):997-998.
[Crossref] [Google Scholar] [Pubmed]
- Al Hajj GN, Haddad J. Preventing staple-line leak in sleeve gastrectomy: Reinforcement with bovine pericardium vs. oversewing. Obes Surg 2013;23(11):1915-1921.
[Crossref] [Google Scholar] [Pubmed]
- Elward ASM, Fahmy MHA, Abu-Seida AM. Greater curvature as a gastric pouch for sleeve gastrectomy: A novel bariatric procedure. Feasibility study in a canine model. Surg Obes Relat Dis 2018;14(12):1814-1820.
[Crossref] [Google Scholar] [Pubmed]
- Natoudi M, Theodorou D, Papalois A, Drymousis P, Alevizos L, Katsaragakis S, et al. Does tissue ischemia actually contribute to leak after sleeve gastrectomy? An experimental study. Obes Surg 2014;24(5):675-683.
[Crossref] [Google Scholar] [Pubmed]
- Genc V, Sulaimanov M, Kırımker EO, Sevim Y, Ensari C. The use of porcine acellular dermal matrix for management of gastrocutaneous fistula after laparoscopic sleeve gastrectomy. J Laparoendosc Adv Surg Tech A 2015;25(5):401-405.
[Crossref] [Google Scholar] [Pubmed]
Copyright: © 2023 The Authors. This is an open access article under the terms of the Creative Commons Attribution Non Commercial Share Alike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/) This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.