Research Article - Archives of Clinical and Experimental Surgery (2022)
Preservation of the Tenon's Capsule and Sub-Tenon's Space after the Autologous Lim-bal-Conjunctival Transplantation in Pterygium Surgery
Fernanda S Vidal*, Sebastiao Cronemberger and Jose Aloisio MassoteFernanda S Vidal, Department of Ophthalmology and Otorhinolaryngology, Federal University of Minas Gerais, Belo Horizonte, Brazil, Email: oftalmoceme@gmail.com
Received: 07-Feb-2022, Manuscript No. EJMACES-22-53458; Editor assigned: 09-Feb-2022, Pre QC No. EJMACES-22-53458; Reviewed: 23-Feb-2022, QC No. EJMACES-22-53458; Revised: 28-Feb-2022, Manuscript No. EJMACES-22-53458; Published: 07-Mar-2022
Abstract
Purpose: No previous studies have evaluated the scarring of the conjunctiva and Tenon’s Capsule (TC) separately when only the conjunctiva is removed, and TC is preserved in the upper part of the bulb: the site designated for trabeculectomy. Thus, this cohort interventional study aims to investigate the scarring of the donor area after Autologous Limbal-Conjunctival Transplantation (ALCT) using Anterior Segment Optical Coherence Tomography (AS-OCT).
Methods: Twenty-three eyes with pterygium were submitted to resection and local reconstruction with ALCT. AS-OCT images obtained from each eye preoperatively served as postoperative control. ALCT was removed superiorly with preservation of TC. The cure of this donor area occurred by secondary intention. AS-OCT was performed preoperatively and 30 and 180 days postoperatively in the donor area, measuring the thickness of the conjunctiva (epithelium and stroma) and TC. The Sub-Tenon’s Space (STS) was clinically assessed.
Results: The mean thickness of the conjunctival epithelium was 48.04 μm ± 11.37 μm in the preoperative period and 51.87 μm ± 15.04 μm 180 days after surgery, without statistically significant difference (P=0.282). A statistically significant increase (P=0.005) in the mean thickness of the conjunctival stroma, from 85.35 μm ± 23.10 μm in the preoperative period to 101.61 μm ± 20.19 μm 180 days after surgery was found. TC had no significant changes, slightly increasing from 117.13 μm ± 24.26 μm preoperatively to 118.09 μm ± 19.24 μm (P=0.808) 180 days after surgery. STS was found in 19 (82%) eyes 180 days after surgery.
Conclusion: Following ALCT with TC preservation, scarring took place in the conjunctiva, epithelium, and stroma. The TC and the STS were preserved not showing any significant changes.
Keywords
Conjunctiva; Tenon’s capsule; OCT; Trabeculectomy; Sub- tenon’s space
Background
Pterygium is one of the most prevalent eye diseases in tropical regions [1]. It is mainly treated with surgery, and its recurrence is reduced via an ALCT [2-4]. The donor area for ALCT is the superior bulbar conjunctiva, region often used in Trabeculectomy (TRAB). TRAB creates a new pathway for aqueous humor drainage. The aqueous humor is diverted into the STS via a trabecular-scleral fistula [5,6]. Closure of this fistula by fibrotic tissue is the most common cause of TRAB failure [7]. Several studies have focused on the scarring of the conjunctiva and TC, characteristically a mobile connective tissue situated below the conjunctiva [5,8-12]. TC has a dense amorphous histological structure and contains fibroblasts [13-17]. Being one connective tissue, TC heals completely by fibrosis, [7,14,18-20] compromising its mobility and providing poor TRAB outcomes. Furthermore, the TC adheres only weakly to the episclera, and is separated from it by the STS [20].
Regarding the increased risk of TRAB failure in eyes with a history of surgery, this cohort interventional study aims to assess, using AS-OCT, scar evolution in the donor superior bulbar conjunctiva, TC, and STS, of which the latter two are not excised during ALCT [12,21,22]. From the best of our knowledge, this work is the first to assess the preservation of TC and STS at the tissue donor site.
Methods
All patients with pterygium examined in our office between May 2017 and May 2018 were invited to participate. Surgical procedures were performed by the same surgeon (FSV) during the same period. The inclusion criteria were age over 25 years and primary or recurrent pterygium in eyes with an intact superior limbal-conjunctival donor area. No restrictions were placed in the length of the pterygium. Exclusion criteria were recurrent pterygium in which the superior limbal-conjunctival region had been used as a donor in a previous surgery; eyes with diseases other than pterygium; very small or asymptomatic pterygium; patients with diffuse or sectoral limbic failure involving the superior limbal area; chronic inflammation from other etiologies not related to the pterygium itself; patients who failed to attend all postoperative assessments. The study followed the principles of the 1964 Declaration of Helsinki and was approved by the Research Ethics Committee of Federal University of Minas Gerais (CAAE: 80402017.4.0000.5149). All participating patients read and signed the previously approved informed consent form.
Surgical technique
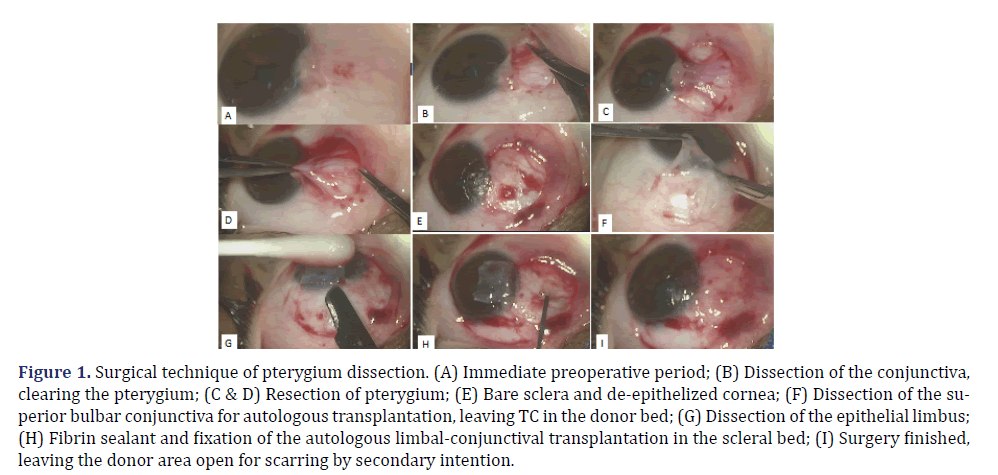
Patients underwent wide pterygium dissection, clearance of the limbus, and resection of the pterygium’s fibrovascular bundle (Figures 1A, 1B, 1C, 1D and 1E). The tissue for ALCT was removed from the superior bulbar conjunctiva. This graft had the same dimensions as the clear limbal and scleral areas at the site of the pterygium resection, as verified using a millimeter compass (Figures 1F and 1G). Only the conjunctiva and limbal epithelium were removed, avoiding manipulation and excision of the TC as much as possible (Figures 1F and 1G). The donor area was neither sutured nor cauterized nor had its wound edges approximated (Figure 1H). The clear limbal and nasal sclera was restored after pterygium removal with ALCT, which was fixed using a fibrin sealant (Tyssel®; Baxter AG, Vienna, Austria) [23] (Figures 1H and 1I).
Figure 1. Surgical technique of pterygium dissection. (A) Immediate preoperative period; (B) Dissection of the conjunctiva, clearing the pterygium; (C & D) Resection of pterygium; (E) Bare sclera and de-epithelized cornea; (F) Dissection of the superior bulbar conjunctiva for autologous transplantation, leaving TC in the donor bed; (G) Dissection of the epithelial limbus; (H) Fibrin sealant and fixation of the autologous limbal-conjunctival transplantation in the scleral bed; (I) Surgery finished, leaving the donor area open for scarring by secondary intention.
Clinical evaluation in slit lamp and digital photography
Patients were clinically evaluated pre- and postoperatively by the same surgeon (FSV) for the biomicroscopic variables in the superior part of their eyeballs. In the pre- and postoperative visits (1, 7, 30, and 180 days after surgery), photographs of donor area were taken without flash at 10x and 16x magnification using a camera (Nikon®; Nikon Imaging Japan Inc.) attached to a slit-lamp (HR Asapt;® São Paulo, Brazil). We assessed: (1) conjunctival hyperemia (present or absent); (2) vessel path alteration in the donor area (congestion, path distortion, and variation in the number of vessels; their presence or absence, only considered ≥ 7 days after surgery.
Assessment of conjunctival mobility
It was assessed by the surgeon (FSV) using a cotton swab after instilling eye drops of 0.5% proxymetacaine (Anestalcon®; Alcon®, São Paulo-SP, Brazil). Conjunctiva was slightly pressed and displaced in the vertical and horizontal directions. Mobility was present when tissue displacement was seen. Data was recorded in a specific research protocol.
Assessment by external examiners
Six months after surgery, another clinical slit-lamp assessment of patients was performed by two ophthalmologists, glaucoma specialists, with over ten years of experience in TRAB. They were unaware of the preoperative presentation of patients and the scarring evolution up to examination. They answered questions about (1) observation of blood vessels from the ocular surface towards the limbus (presence or absence of changes such as congestion, path distortion, and variation in the number of vessels), (2) tissue mobility in the donor and recipient areas (presence or absence, assessed by touching with a cotton swab, and (3) the viability of surgical reintervention at the site, including TRAB.
AS-OCT evaluation
AS-OCT exams were performed one day before the pterygium excision and, 30 and 180 days afterwards, using the Optovue-Avanti® device (Optovue Inc., Fremont, California, USA) with the lens as the anterior segment. This device acquires images at a speed of 70,000 A-scans/second and an axial resolution of 5 μm [24]. Conjunctival thickness and TC measurements were assessed based on the study by Howlett et al. who evaluated the conjunctiva-Tenon’s capsule complex in the superior bulbar conjunctiva [25].
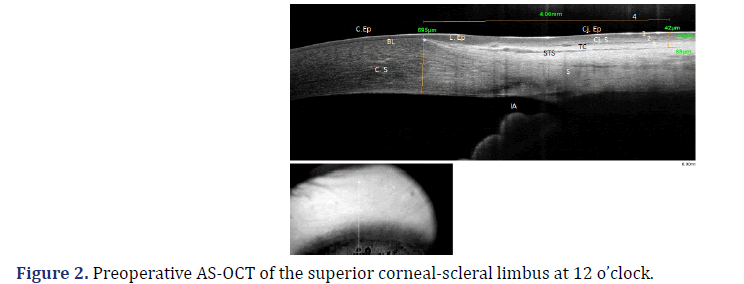
The following pre- and postoperative measurements were compared (Figure 2): (1) the thickness of the conjunctival epithelium; (2) the thickness of the conjunctival stroma; (3) the thickness of the TC, and (4) the assessment of the presence or absence of the STS.
BL: Bowman’s Layer; C.Ep: Corneal Epithelium; C. S: Corneal Stroma; Cj. Ep: Conjunctiva Epithelium; Cj. S: Conjunctiva Stroma; TC: Tenon’s Capsule; STS: Sub-Tenon’s Space; S: Sclera; IA: Iridocorneal Angle, on the left, image of the area of the eyeball where the cross section is performed.
*Marking the beginning of the epithelial limbus and the end of the Bowman’s Layer (corneal thickness at this site: 695μm); (1) Thickness of the conjunctival epithelium: 42μm; (2) Thickness of the conjunctival stroma: 60 μm; (3) Thickness of Tenon’s capsule: 68μm; (4) Measuring 4 mm after the beginning of the limbus.
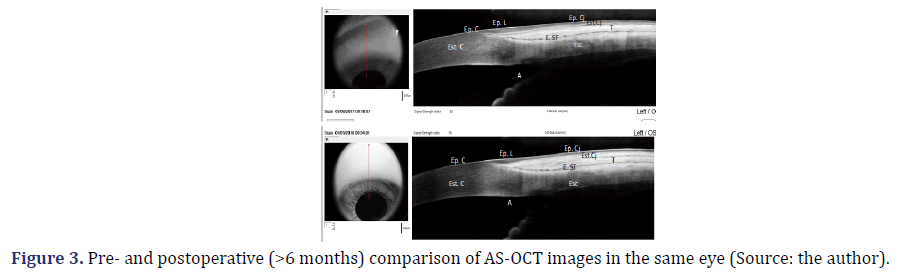
To determine the site where TRAB trans-scleral fistula normally drains the aqueous humor, usually 3 mm from the limbus, the beginning of the epithelial limbus was marked, coinciding with the end of Bowman’s layer [26]. This point of reference is accurate, unlike the transition from the epithelium limbus to the conjunctival epithelium, which is not a specific anatomical marker reproducible in all eyes. Measurements were taken 4 mm from the beginning of the limbus, instead of 3 mm from the end of the limbus (Figure 2). These measurements were performed using the imaging software built into the device. Each structure of interest was determined, and the thickness was recorded. The inbuilt software also obtained comparative AS-OCT pre- and postoperative images (Figure 3).
C.Ep: Corneal Epithelium, C. S: Corneal Stroma, Cj. Ep: Conjunctival Epithelium, Cj. S: Conjunctiva Stroma, TC: Tenon’s Capsule, STS: Sub-Tenon’s Space, S: Sclera, IA: Iridocorneal Angle on the left, image of the area of the eyeball where the cross section is performed.
(Preoperative period on the top; Postoperative period on the bottom) The continuity of the epithelium of the conjunctiva, limbus, and cornea was observed in the preoperative and postoperative periods, maintaining the pattern of hyporeflectivity. The conjunctival stroma had a higher reflectivity in the postoperative period over 180 days after surgery. The Tenon’s capsule and the sub-Tenon’s space had the same reflectivity pattern in the preoperative and postoperative periods.
Sample calculation: Include size, getting sample size calculation
Sample calculation was determined using the conjunctival epithelium and conjunctival/TC stromal thickness from our pilot study, which used data from the first 10 patients and had a normal distribution. Standard deviation values of the conjunctival epithelium and conjunctival/TC stromal thickness were 7.35 μm and 32.61 μm, respectively. These values were similar to those reported by Zhang et al. [27,28] involving AS-OCT of the bulbar conjunctiva. Thus, the standard deviation values considered for the sample calculation were those obtained by Zhang et al. of a conjunctival epithelial thickness of 7.4 μm and a conjunctival/TC stromal set of 32.5 μm. With a sample of at least 23 eyes, a statistical power of 90% would be obtained in detecting the differences when comparing the pre- and postoperative mean values of the conjunctival epithelial thickness, as well as of the stromal/TC complex.
Statistical analysis
A descriptive analysis was performed by evaluating the mean and the standard deviation of variables with normal distributions. The Shapiro–Wilk test was used to verify the hypothesis of normality for continuous variables in Micrometers (μm). Paired Student’s t-test was used to compare the mean pre- and postoperative measurements of conjunctival epithelium thickness and the conjunctiva/TC stromal complex thickness.
Analyses were performed using the SPSS® Software (IBM, Chicago, IL) version 18, with a level of statistical significance of P<0.05.
Results
Thirty-two patients agreed to participate, but only 22 had all exams and postoperative assessments, yielding a total of 23 eyes (one patient had both eyes operated). Ten patients were excluded because they did not complete the follow-up. Among patients, 65% were female and 12% had recurrent pterygium. Patients’ age ranged from 31 to 67 years, mean age of 49.0 ± 11.8.
Clinical evaluation and anterior segment photographs
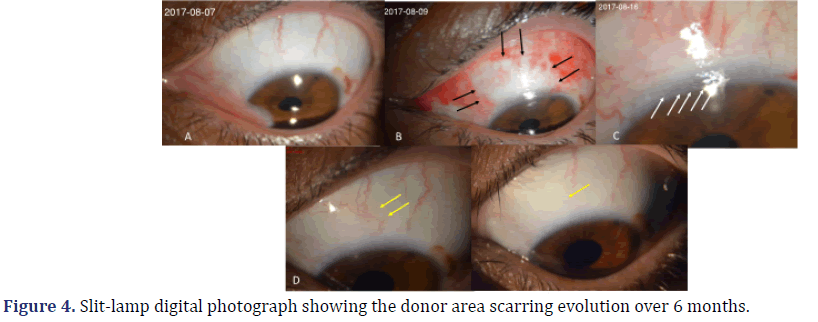
In the preoperative period (Figure 4A), conjunctival hyperemia was present in two (8.6%) eyes, which had large pterygia and recurrent chronic inflammation. Conjunctival hyperemia was seen in all 23 (100%) eyes, one to seven days after surgery (Figures 4B and 4C). Thirty days after surgery, conjunctival hyperemia was seen in only 16 (69.5%) eyes (Figure 4D). After 180 days, it was seen in only two (8.6%) eyes (Figure 4E).
(A) preoperative; (B) 1st day after surgery (black arrows show the edges of the conjunctival incision); (C) 7th day after surgery: delimitation of the limbus (white arrows) and conjunctival area is seen, with tissue congestion and changes of vessels; (D) 30th day after surgery: delimitation of the donor area is not evident, and changes in the vascular path, compared to the preoperative period, are indicated by yellow arrows; (E) 180th day after surgery: restoration of the epithelium and vessels with a similar appearance to the preoperative period.
Vascular changes
It was analyzed seven days after surgery. Vascular changes were compared between pre- and postoperative photographic images from the donor area. Vascular changes were seen in 22 (95.6%) eyes seven days after surgery (Figure 4C). Thirty days after surgery, they were seen in 23 (100%) eyes (Figure 4D). After 180 days, they were seen in six (26%) eyes, while the remaining 17 (74%) had a vascular path similar to their respective preoperative one (Figure 4E).
External examiners
Examiners 1 and 2 observed that, respectively, 21 (91.3%) and 22 (95.6%) eyes had no changes in the radial path of vessels (Table 1, column 2). For examiner 1, the feasibility of successful surgical intervention, including TRAB, would be possible in 22 (95.6%) eyes (Table 1, column 3) and unfeasible in only one (4.4%) eye. For examiner 2, all 23 (100%) eyes were considered as feasible for successful surgical intervention. Regarding the mobility of the TC-conjunctiva complex, both examiners agreed that 100% of the eyes had that mobility preserved (Table 1, column 4).
| Examiner | Alteration of the radial path of the vessels | Feasibility for surgical reintervention | Mobility of the Tenon-conjunctive set | |||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| 1 | 2 (8,7%) |
21 (91,3%) |
22 (95,6%) | 1 (4,4%) |
23 (100%) |
0 |
| 2 | 1 (4,4%) |
22 (95,6%) |
23 (100%) | 0 |
23 (100%) |
0 |
Absolute values and percentages of responses obtained after evaluation by the external examiners.
AS-OCT evaluation
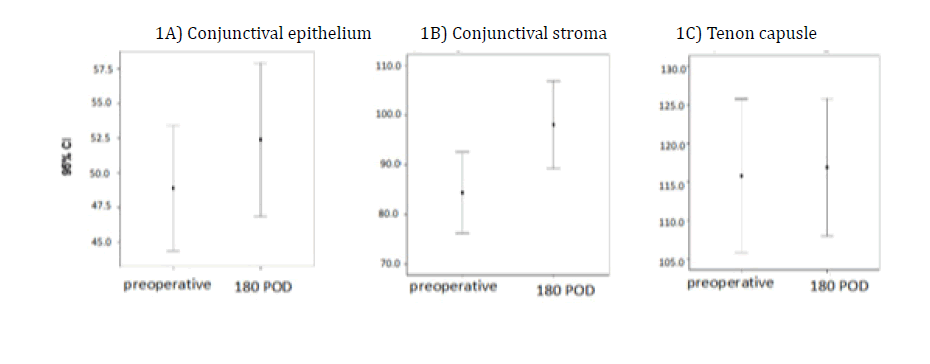
We made a statistical comparison between the mean thicknesses of the conjunctival epithelium, conjunctival stroma, and TC preoperatively with those 180 days after surgery (Figure 5). The same comparison was made using charts between the mean thickness in the preoperative and postoperative periods over 6 months (Figures 5A, 5B and 5C). Figure 5A shows a small variation in the means, with an overlapping confidence interval. There was no statistically significant difference (P=0.282) between the mean conjunctival epithelium measurements in the preoperative (48.04 μm ± 11.37 μm) and postoperative periods (51.87 μm ± 15.04 μm).
Figure 5B shows a significant difference between the means of the pre- and postoperative measures of the conjunctival stroma (P=0.005). An increase is seen in the mean from 85.35 μm ± 23.10 μm in the preoperative period to 101.61 μm ± 20.19 μm after six months. Figure 5C shows a difference in the mean TC thickness in the preoperative period (117.13 μm ± 24.26 μm) and that measured 180 days after surgery (118.09 μm ± 19.24 μm). This increase in the postoperative period did not have a statistically significant difference (P=0.808). These anatomical structures were assessed for changes in the presence or absence of the STS. In the postoperative period, the STS were preserved in 19 (82.6%) eyes.
A) shows no statistical significance difference (p=0.282) in the mean conjunctival epithelium measurements in the pre and postoperative period; B) It was observed that there was a statistically significant difference (p<0.008) in the comparison between pre- and postoperative thickness measures of the conjunctival stroma; C) The mean thickness of the Tenon capsule, pre- and postoperatively, showed no significant difference (p=0.808), with the confidence interval being very close between them.
Discussion
In the present study, clinical assessments were performed to identify signs of fibrosis or scar distortion in the region of ALCT. Hyperemia in the donor area and vascular changes, which were seen during the scarring from seven to 30 days after surgery in all 23 eyes, did not persist after 180 days after surgery. This suggests that its occurrence was due to the scarring inflammatory phase in the donor conjunctiva, without residual fibrosis after the end of scarring. The mobility of the conjunctiva-TC complex was normal in all eyes in the pre- and postoperative period in the donor area. This preservation of tissue mobility indirectly indicated that TC and STS showed no scarring adhesions to episclera and sclera. Therefore, it is important to highlight that the healing of the donor area occurs by secondary intention, without approaching its edges.
The external examiners
When evaluating the donor area six months after ACLT, external examiners identified no signs of fibrosis, except for one patient who had the largest pterygia. The donor area was considered feasible for surgical reintervention, including TRAB, in all eyes, except for one eye.
AS-OCT
The images measured pre- and postoperatively by AS- OCT, showed preserved TC and STS in most patients. Changes in thickness and reflectivity only occurred in the conjunctival stroma in most eyes. Consequently, this suggests that the inflammatory scarring reaction occurred in the conjunctival stromal level without compromising the deep levels. Comparing clinical findings with those obtained by AS-OCT, it is observed that there exists correlation of the preserved conjunctiva-Tenon’s mobility. By preserving TC, the fibrous reaction of the deep layers is prevented, and therefore, its preservation acts as a shield for the STS. Thus, the three tissue levels above the STS, as seen in AS-OCT, showed distinct behaviors after conjunctival donation. The conjunctival epithelium regenerated through its stem cells, resuming its preoperative appearance and measurements. The conjunctival stroma showed changes resembling fibrosis, such as the increase of its reflectivity and its thickness. The TC remained unchanged in most eyes. Preservation of the TC in the perioperative period probably prevented the inflammatory scar response in this tissue as well as fibrosis and adhesions to episclera, and also preserving the STS. Given that the superior bulbar region must scar well to allow for surgical treatment of glaucoma, it is not surprising that several studies have addressed this topic [5,7,8,10,12,29,30]. However, most of these studies do not clearly distinguish between the conjunctiva and the TC. The TC was often identified as subepithelial tissue or subconjunctival space [5,10]. Nevertheless, the trans-scleral fistula produced by TRAB releases aqueous humor into the sub-Tenon’s region, not into the subconjunctival region [6]. In other words, the aqueous humor is released between the TC and episclera and not between the conjunctiva and TC. In studies analyzing the association between TRAB failure and the previous surgery, surgeries involved the incision of the conjunctiva, the TC, and the episclera, leading to fibrotic adhesions in the TC and episclera and there is no STS in the area where the surgery was performed [8,10,31]. Broadway et al. concluded that TRAB failure was more common in patients undergoing procedures after previous conjunctival management [10]. However, surgeries considered by these authors in 1998, such as cataract and retinopexy, were trans-scleral procedures. They did not distinguish between structures and considered conjunctiva and CT, collectively, as the conjunctiva. Gozawa et al. [31] used AS-OCT in the superior bulbar region in patients submitted to trans-scleral cataract removal, using the access to the lens through the superior bulbar region in half the patients and temporal access in the other half. They compared the procedure performed in rabbit eyes, which were later submitted to histological sectioning of the superior bulbar region in the pre- and postoperative periods. Structural changes were seen in the conjunctiva and TC after the superior trans-scleral incision in the AS-OCT, as well as inflammatory findings in the histological evaluation. In the eyes with temporal incisions, the histological sections of the superior bulbar region, which was not operated upon, showed no inflammatory signs. AS-OCT has shown that untouched tissues had been preserved. Thus, Gozawa et al. concluded that the TRAB altered the connective tissue and TC and that these findings were not seen with temporal incisions, preserving the superior bulbar region. Nevertheless, the surgical technique for a trans-scleral phakectomy was performed within the conjunctiva, TC, STS, and sclera [31]. Furthermore, the AS-OCT images were collected using Casia SS-1000® (Tomey Corporation, Nagoya-Aichi-Japan), which has an axial resolution of 10 μm half of the AS-OCT resolution used in the present study [24]. Thus, in the study of Gozawa et al., images had a lower resolution, making it difficult to differentiate the structures. In contrast to the findings of Gozawa et al., our results showed that by preserving the TC and STS during surgery it was possible to maintain their reflectivity and thickness during the postoperative period. Previous studies involving restrictive syndrome were also considered. This syndrome is seen during the postoperative period in patients with strabismus or retinal detachment treated with scleral fixation. During the scleral fixation, TC was excessively manipulated with extensive muscle dissection [18,32,33], with fibrotic restriction of TC and its adherence to episclera [32]. As a preventive measure for this surgical complication, these studies recommended minimal TC manipulation during such procedures [34]. Comparing analysis of this surgical complication with our findings, we must highlight the importance of TC preservation for maintaining the conjunctiva-TC complex mobility. Studies examining TC fibroblasts have provided a better understanding of the development of the fibrosis responsible for TRAB failure [7,16,34-36]. In an animal model of scarring after a fistulizing surgery, fibroblasts were seen to arise from episclera and the subconjunctival connective tissue in a study conducted by Joseph et al. [11]. In other words, they come from the structures that delimit the STS, the TC and episclera, as well as from sites neighboring muscle emergence [11], demonstrating the importance of these structures in the fibrosis of the STS. Therefore, our findings corroborate the literature regarding the importance of preserving the TC in order to avoid postoperative fibrosis. In studies that investigated the scarring of the conjunctiva, TC, and STS separately [21,31], surgical procedures caused injuries in all of these structures, without any attempts of preservation, since procedures involved trans-scleral incisions. The conjunctival excision with TC preservation allows for full scarring similar to that of the preoperative state, both with the slit lamp photography and with AS-OCT. In addition, the procedure preserves the STS, maintaining tissue mobility without adhesions to the episclera. These findings are important for patients undergoing surgical treatment of the ocular surface. This is particularly important for those who need multiple procedures in the same region, such as those with pterygium and glaucoma. Further studies evaluating surgical reintervention in the donor area and the following scarring process should be performed to assess the effectiveness of TRAB after donating conjunctival tissue with the preservation of TC.
Conclusions
After ALCT with TC preservation, scarring occurs in the conjunctiva, epithelium, and stroma. The underlying TC and the STS structures remain preserved for eventual surgery.
Key Messages
• Successful trabeculectomy is more likely in eyes in which the conjunctiva and Tenon’ capsule is normal.
• The present study is the first to evaluate the healing of the conjunctiva and the Tenon´s capsule separately, in the upper part of the bulb. When the conjunctiva was resecting and Tenon’s capsule was preserved. Considering also the aspect of the sub-Tenon´s space. These structures were studied using high-resolution AS-OCT.
• Healing occurred in the conjunctiva (epithelium and stroma), while the Tenon´s capsule and the sub-Tenon’s space were preserved.
Declarations
Funding
No funds, grants, or other support was received. Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest/competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Fernanda S. Vidal and Sebastião Cronemberger conceived and designed the protocol. All authors were involved in the analysis and interpretation of the data. FSV, SC and JAM wrote the first draft of the manuscript, revised the manuscript and produced the final version. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
This study followed the principles of the 1964 Declaration of Helsinki and was approved by the Research Ethics Committee of Federal University of Minas Gerais (CAAE: 80402017.4.0000.5149). All participating patients read and signed the previously approved informed consent form.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The participant has consented to the submission of the article to the journal.
Patients signed informed consent regarding publishing their data and photographs.
Acknowledgements
We wish to thank the external examiners: Dr. Heloísa Maestrini and Dr. Herika Danielle de Miranda Santos, who carefully assessed each patient; Dr. Moisés Salgado Pedrosa, the anatomy-pathologist, for his important collaboration in the analysis of AS-OCT images, and Oculare Ophthalmology Hospital particularly Dr. Angela Maestrini, who helped in all stages of this work.
Abbreviations
CDK: Cyclin Dependent Kinase; USFDA: United States Food And Drug Act; HER: Human Epidermal Growth Factor Receptor; AI: Aromatase Inhibitor; NSAI: Non-Steroidal Aromatase Inhibitor; HCl: Hydrogen Chloride; DCM: Dichloromethane; N2: Nitrogen(g); SNAr: Substituted Nucleophilic Aromatic; SMB: Simulated Moving Bed; API: Active Pharmaceutical Ingredient; ICH: International Conference on Harmonization; MeOH: Methanol; THF: Tetrahydrofuran; DIPEA: Diisopropyl Ethylamine; DMAP: 4-Dimethylaminopyridine; DABCO: 1,4-Diazabicyclo(2.2.2)octane; NBS: N-Bro-mo Succinimide; NMP: N-Methyl Pyrrolidine; K2 CO3 : Potassium Carbonate; Cs2CO3 : Caesium Carbonate; CsF: Caesium Fluoride; KF: Potassium Fluoride; TEA: Triethylamine; DCHA: Dicyclohexylamine; n-BuOH: n-Butanol; NMR: Nuclear Magnetic Resonance; DMF: Dimethyl Formamide; DMSO: Dimethyl Sulfoxide; R.T: Room Temperature; HPLC: High Pressure Liquid Chromatography; NaOH: Sodium Hydroxide; DMAc: Dimethyl Acetamide; TFAA: Trifluoroacetic acid; EDC: Dichloroethane; XRD: X-ray Diffraction; DSC: Differential Scanning Calorimetry; Mg: Milligram; NaCN: Sodium Cyanide; Boc2O: Boc-anhydride; MIBK: Methyl isobutyl ketone; °C: Degree Celsius; i-PrOH: Isopropanol; EtOAc: Ethyl Acetate; Hex: Hexane; NSCLC: Non-Small Cell Lung Cancer; MtBE: Methyl tert. Butyl Ether; Na2 CO3 : Sodium Carbonate; DME: Dimethoxyethane; PCl3 : Phosphoryl Chloride; HNO3 : Nitric Acid; HCOOH: Formic Acid; RH: Relative Humidity
References
- Liu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk factors for pterygium: A systematic review and meta-analysis. BMJ open 2013; 3(11):e003787.
[Crossref] [Google Scholar] [pubmed]
- Clearfield E, Muthappan V, Wang X, Kuo IC. Conjunctival autograft for pterygium. Cochrane Database of Systematic Reviews 2016(2).
[Crossref] [Google Scholar] [Pubmed]
- Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 2002;109(9):1752-5.
[Crossref] [Google Scholar] [Pubmed]
- Al Fayez MF. Limbal-conjunctival vs conjunctival autograft transplant for recurrent pterygia: a prospective randomized controlled trial. JAMA ophthalmology 2013;131(1):11-6.
[Crossref] [Google Scholar] [Pubmed]
- Yamanaka O, Kitano-Izutani A, Tomoyose K, Reinach PS. Pathobiology of wound healing after glaucoma filtration surgery. BMC ophthalmology 2015;15(1):19-27.
[Crossref] [Google Scholar] [Pubmed]
- Razeghinejad MR, Fudemberg SJ, Spaeth GL. The changing conceptual basis of trabeculectomy: a review of past and current surgical techniques. Surv Ophthalmol 2012;57(1):1-25.
[Crossref] [Google Scholar] [Pubmed]
- Stahnke T, Löbler M, Kastner C, Stachs O, Wree A, Sternberg K, et al. Different fibroblast subpopulations of the eye: a therapeutic target to prevent postoperative fibrosis in glaucoma therapy. Exp Eye Res 2012;100: 88-97.
[Crossref] [Google Scholar] [Pubmed]
- Cordeiro MF, Chang L, Lim KS, Daniels JT, Pleass RD, Siriwardena D, et al. Modulating conjunctival wound healing. Eye 2000;14(3):536-47.
[Crossref] [Google Scholar] [Pubmed]
- Zada M, Pattamatta U, White A. Modulation of fibroblasts in conjunctival wound healing. Ophthalmology 2018;125(2):179-92.
[Crossref] [Google Scholar] [Pubmed]
- Broadway DC, Grierson I, Hitchings RA. Local effects of previous conjunctival incisional surgery and the subsequent outcome of filtration surgery. Am J ophthalmol 1998;125(6):805-18.
[Crossref] [Google Scholar] [Pubmed]
- Joseph JP, Miller MH, Hitchings RA. Wound healing as a barrier to successful filtration surgery. Eye(Lond) 1988;2(1):S113-23.
[Crossref] [Google Scholar] [Pubmed]
- Schlunck G, Meyer-ter-Vehn T, Klink T, Grehn F. Conjunctival fibrosis following filtering glaucoma surgery. Exp eye res 2016;142:76-82.
[Crossref] [Google Scholar] [Pubmed]
- Zhang JY, Gao P, Ye W, Xiao YQ. Functional characteristics of connective tissue growth factor on human tenon’s capsule fibroblast. Curr Eye Res 2014;39(1):53-61.
[Crossref] [Google Scholar] [Pubmed]
- Kakizaki H, Takahashi Y, Nakano T, Asamoto K, Ikeda H, Ichinose A, et al. Anatomy of Tenons capsule. Clin Exp Ophthalmol 2012 Aug;40(6):611-6.
[Crossref] [Google Scholar] [Pubmed]
- Shauly Y, Miller B, Lichtig C, Modan M, Meyer E. Tenon's capsule: Ultrastructure of collagen fibrils in normals and infantile esotropia. Invest Ophthalmol Vis Sci 1992;33(3):651-6.
[Google Scholar] [Pubmed]
- Erol YO, Atilla P, Acaroglu G, Muftuoglu S, Karakaya J. A histopathological investigation of Tenon’s capsule in diabetic eyes. Int Ophthalmol 2017;37(3):627-33.
[Crossref] [Google Scholar] [Pubmed]
- Wright KW. The fat adherence syndrome and strabismus after retina surgery. Ophthalmology. 1986;93(3):411-5
[Crossref] [Google Scholar] [Pubmed]
- Rockey DC, Bell PD, Hill JA. Fibrosis—A common pathway to organ injury and failure. N Engl J Med 2015;372(12):1138-49.
[Crossref] [Google Scholar] [Pubmed]
- Kumar DA, Agarwal A, Karnathi S, Patadiya R. Anterior segment optical coherence tomography for imaging the sub-Tenon space. Ophthalmic Res 2013;50(4):231-4.
[Crossref] [Google Scholar] [Pubmed]
- Gozawa M, Takamura Y, Miyake S, Iwasaki K, Arimura S, Takihara Y, et al. Comparison of subconjunctival scarring after microincision vitrectomy surgery using 20‐, 23‐, 25‐and 27‐gauge systems in rabbits. Acta ophthalmologica 2017;95(7):e602-9.
[Crossref] [Google Scholar] [Pubmed]
- Baradaran-Rafii A, Eslani M, Jamali H, Karimian F, Tailor UA, Djalilian AR, et al. Postoperative complications of conjunctival limbal autograft surgery. Cornea 2012;31(8):893-9.
[Crossref] [Google Scholar] [Pubmed]
- Jiang J, Yang Y, Zhang M, Fu X, Bao X, Yao K, et al. Comparison of fibrin sealant and sutures for conjunctival autograft fixation in pterygium surgery: one-year follow-up. Ophthalmologica 2008;222(2):105-11.
[Crossref] [Google Scholar] [Pubmed]
- Venkateswaran N, Galor A, Wang J, Karp CL. Optical coherence tomography for ocular surface and corneal diseases: A review. Eye and Vision 2018;5(1):1-1.
[Crossref] [Google Scholar] [Pubmed]
- Howlett J, Vahdani K, Rossiter J. Bulbar conjunctival and Tenon's layer thickness measurement using optical coherence tomography. J Curr Glaucoma Pract 2014;8(2):63.
[Crossref] [Google Scholar] [Pubmed]
- Gonzalez G, Sasamoto Y, Ksander BR, Frank MH, Frank NY. Limbal stem cells: Identity, developmental origin, and therapeutic potential. Wiley Interdiscip Rev Dev Biol 2018;7(2):e303.
[Crossref] [Google Scholar] [Pubmed]
- Zhang X, Li Q, Xiang M, Zou H, Liu B, Zhou H, et al. Bulbar conjunctival thickness measurements with optical coherence tomography in healthy Chinese subjects. Invest Ophthalmol Vis Sci 2013;54(7):4705-9.
[Crossref] [Google Scholar] [Pubmed]
- Zhang X, Li Q, Liu B, Zhou H, Wang H, Zhang Z, et al. In vivo cross-sectional observation and thickness measurement of bulbar conjunctiva using optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52(10):7787-91.
[Crossref] [Google Scholar] [Pubmed]
- Yu DY, Morgan WH, Sun X, Su EN, Cringle SJ, Paula KY, et al. The critical role of the conjunctiva in glaucoma filtration surgery. Prog Retin Eye Res 2009;28(5):303-28.
[Crossref] [Google Scholar] [Pubmed]
- Francis BA, Du LT, Najafi K, Murthy R, Kurumety U, Rao N, et al. Histopathologic features of conjunctival filtering blebs. Arch Ophthalmol 2005; 123(2):166-70.
[Crossref] [Google Scholar] [Pubmed]
- Gozawa M, Takamura Y, Miyake S, Yokota S, Sakashita M, Arimura S, et al. Prospective observational study of conjunctival scarring after phacoemulsification. Acta ophthalmologica 2016;94(7):e541-9.
[Crossref] [Google Scholar] [Pubmed]
- Price RL. Role of Tenon's capsule in postoperative restrictions. Int Ophthalmol Clin 1976;16(3):197-207.
[Crossref] [Google Scholar] [Pubmed]
- Carden SM, Sheth S. Transition from anatomy school to the operating theatre for strabismus surgeons. Clin Exp Ophthalmol 2016;44:854-5.
[Crossref] [Google Scholar] [Pubmed]
- Mojon DS. Review: Minimally invasive strabismus surgery. Eye (Lond) 2015;29: 225-33.
[Crossref] [Google Scholar] [Pubmed]
- Kim D, Pattamatta U, Kelly E, Healey PR, Carnt N, Zoellner H, et al. Inhibitory effects of angiotensin II receptor blockade on human tenon fibroblast migration and reactive oxygen species production in cell culture. Transl Vis Sci Technol 2018;7(2):20.
[Crossref] [Google Scholar] [Pubmed]
- Crowston JG, Akbar AN, Constable PH, Occleston NL, Daniels JT, Khaw PT, et al. Antimetabolite-induced apoptosis in Tenon's capsule fibroblasts. Invest Ophthalmol Vis Sci 1998; 39(2):449-54.
[Google Scholar] [Pubmed]
- Francoz M, Karamoko I, Baudouin C, Labbé A. Ocular surface epithelial thickness evaluation with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52(12):9116-23.
[Crossref] [Google Scholar] [Pubmed]
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.